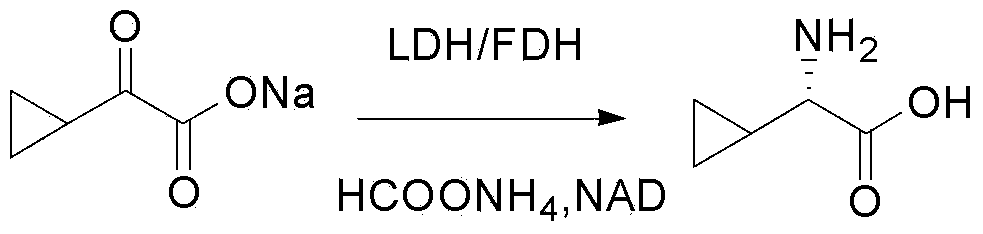L-cyclic alkylamino acid synthesis method and medicinal composition containing L-cyclic alkylamino acid
A technology of alkyl amino acid and synthesis method, which is applied in the field of synthesis method and its pharmaceutical composition, can solve the problems of high price, long synthesis route, large amount of organic solvent, etc., achieve mild reaction conditions, simplify synthesis process, and convert raw materials high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
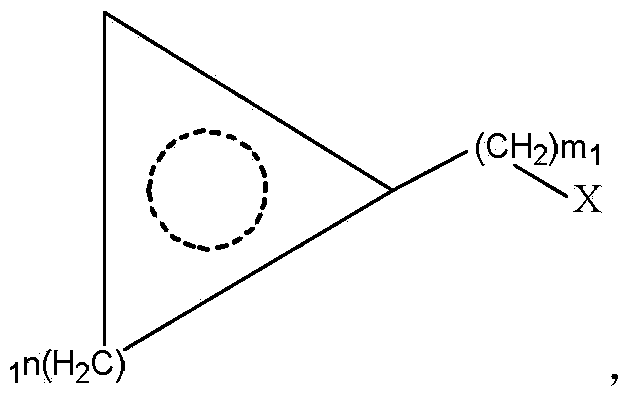
[0029] In a typical embodiment of the present invention, a method for synthesizing an L-cyclic alkyl amino acid is provided, comprising: step A, preparing a cyclic alkyl ketoacid with structural formula (I) or structural formula (II) or Cyclic alkyl ketoacid; step B, combining cyclic alkyl ketoacid or cyclic alkyl ketoacid with ammonium formate, leucine dehydrogenase, formate dehydrogenase and coenzyme NAD + Mixed, carry out reductive amination reaction, generate L-cyclic alkyl amino acid, wherein, structural formula (I) is no 1 ≥1, m 1 ≥0, M 1 Is H or monovalent cation; Structural formula (II) is no 2 ≥0, m 2 ≥0, M 2 It is H or a monovalent cation; the amino acid sequence of leucine dehydrogenase is SEQ ID No.1.
[0030]The above synthesis method utilizes the specific leucine dehydrogenase, formate dehydrogenase and coenzyme NAD whose amino acid sequence is SEQ ID No.1 + Reductive amination reaction of cyclic alkyl keto acids is carried out to generate L-cyclic alky...
Embodiment 1
[0058] Synthesis of L-cyclopropylglycine
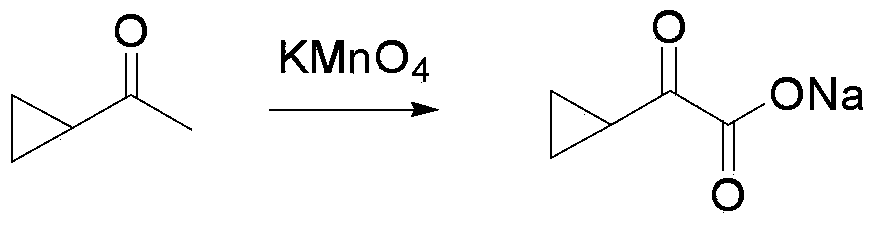
[0059] 1) At room temperature, add 500g cyclopropyl methyl ketone, 15.6g NaOH, and 8.7L water into a 20L four-necked bottle to form a mixed system. After heating the mixed system to 45-55°C, add 8.7L dropwise to the mixed system With 1864.8g KMnO 4 aqueous solution, undissolved KMnO 4 Then add it into the mixed system in batches, after about 10 hours, the reaction tracking raw materials reacted completely, the internal standard yield was 57.9%, and 10% NaHSO was added dropwise to the mixed system after the reaction was completed. 3 Destroy unreacted KMnO 4 ; Suction filtration, the filtrate is concentrated to 70% and then directly used in the next step. 1H NMR (500MHz, CD 3 Cl): δ 1.29 (m, 3H), 1.06 (m, 1H), 0.95 (m, 1H).
[0060]
[0061] 2) Add 1627g of 14.5% sodium cyclopropyloxyacetate aqueous solution, 193.3g of ammonium formate, and 2350mL of leucine dehydrogenase with an enzyme specific activity of 60U / ml to a 5L four-n...
Embodiment 2
[0064] Synthesis of L-cyclobutylglycine
[0065] 1) Add 15.13g of magnesium chips, 160ml of tetrahydrofuran, and 2 iodine particles into a 1L four-necked bottle, and then add 32ml of tetrahydrofuran solution with 8g of bromocyclobutane to it, and heat the four-necked bottle to make bromocyclobutane in the system Initiate with an alkane reagent; then cool the four-necked bottle to 40°C, add dropwise 288ml of tetrahydrofuran solution with 72g of bromocyclobutane to it, and after about 2.5 hours, the bromocyclobutane reacts according to the following reaction formula; The Grignard reagent was obtained after the temperature was kept at 40-50°C for about 1 hour, and the temperature was lowered to room temperature under nitrogen protection for later use.
[0066]
[0067] 2) Add 160ml of tetrahydrofuran solution containing 112.6g of diethyl oxalate into a 1L four-necked flask, and cool the four-necked flask to below -50°C with liquid nitrogen ethanol; The good 0.593mol Grignard ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com