Method for synthesizing Tegrazan chiral alcohol
A technology of chiral reagents and dihydrogen, which is applied in the field of chiral fragment synthesis of raw materials, can solve the problems of poor chiral purity, high cost, and difficulty in purchasing chiral Ru reagent prices, and achieves simple synthesis methods, easy procurement, and chiral Ru reagents. highly selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
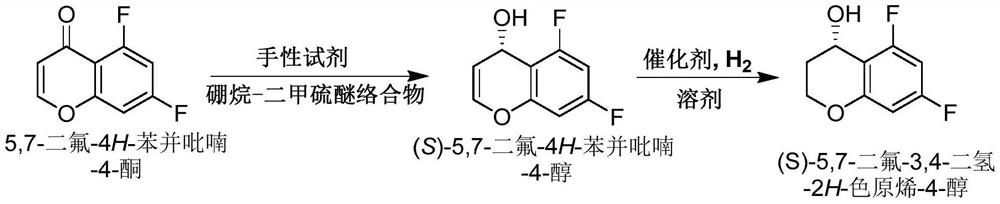
[0023] Example 1: Preparation of (S)-5,7-difluoro-4H-benzopyran-4-ol
[0024] Anhydrous THF (400 mL) and (S)-tetrahydro-1-methyl-,3,3-diphenyl-1H,3H-pyrrolo[1,2-c were added to a 3L reaction flask under nitrogen protection ][1,3,2]oxazaborolane in toluene (1 M in Toluene, 55 mL, 55 mmol). After the addition, the system was cooled to 0±2°C in an ice-salt bath, then borane dimethyl sulfide complex (1M in THF, 575mL, 0.575mol) was added to the system, and the system was stirred at 0±2°C for 10min and then added 5, A solution of 7-difluoro-4H-benzopyran-4-one (100 g, 0.549 mol) in THF (500 mL) was added to maintain the reaction temperature of the system at 0±2 °C. After the addition, the system was reacted to TLC to track the starting material 5,7-Difluoro-4H-benzopyran-4-one disappeared. After completion of the reaction, the system was quenched by adding MeOH (200 mL), and the system was naturally warmed to room temperature and stirred vigorously for 1 hour. The organic solven...
Embodiment 2
[0025] Embodiment 2: the preparation of (S)-5,7-difluoro-4H-benzopyran-4-ol
[0026] Anhydrous THF (80 mL) and (S)-tetrahydro-1,3,3-triphenyl-1H,3H-pyrrolo[1,2-c][1, (S)-tetrahydro-1,3,3-triphenyl-1H,3H-pyrrolo[1,2-c][1, 3,2] oxazaborolane in toluene (1 M in Toluene, 11.0 mL, 11.0 mmol). After the addition, the system was cooled to 0±2° C. in an ice-salt bath, and then borane dimethyl sulfide complex (1M inTHF, 121 mL, 121 mmol) was added to the system. The system was stirred at 0±2°C for 10min, and a solution of 5,7-difluoro-4H-benzopyran-4-one (20.0g, 109.8mmol) in THF (100mL) was added, and the reaction temperature of the system was maintained at 0±2°C during the addition. , after the addition, the system reacted until the TLC tracking material 5,7-difluoro-4H-benzopyran-4-one disappeared. After the completion of the reaction, the system was quenched by adding MeOH (50 mL), and the system was naturally warmed to room temperature and stirred vigorously for 1 hour. The org...
Embodiment 3
[0027] Example 3: Preparation of (S)-5,7-difluoro-3,4-dihydro-2H-chromogen-4-ol
[0028] (S)-5,7-difluoro-4H-benzopyran-4-ol (5.0 g, 27.15 mmol) and ethanol (100 mL) were added to the reaction flask, the system was stirred, and then 5% Pd was added under nitrogen protection / C (65% water content, 5.8 g). After the addition, the system was replaced with nitrogen three times, and then hydrogenated at room temperature and normal pressure until HPLC tracked the disappearance of the reaction raw materials. Filter, remove the organic solvent under high vacuum, and purify the residue by column chromatography (n-heptane / EA=5:1) to obtain (S)-5,7-difluoro-3,4-dihydro-2H-chromene -4-ol (4.65 g, 92%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com