A kind of enone reductase and a kind of preparation method of buvaracetam intermediate
An intermediate, reductase technology, applied in the directions of oxidoreductase, biochemical equipment and methods, enzymes, etc., can solve the problems of no disclosure of alkene reductase information, difficult procurement of substrates, and many reaction steps, etc., and achieve broad industrial application. Promising, easy-to-implement, low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
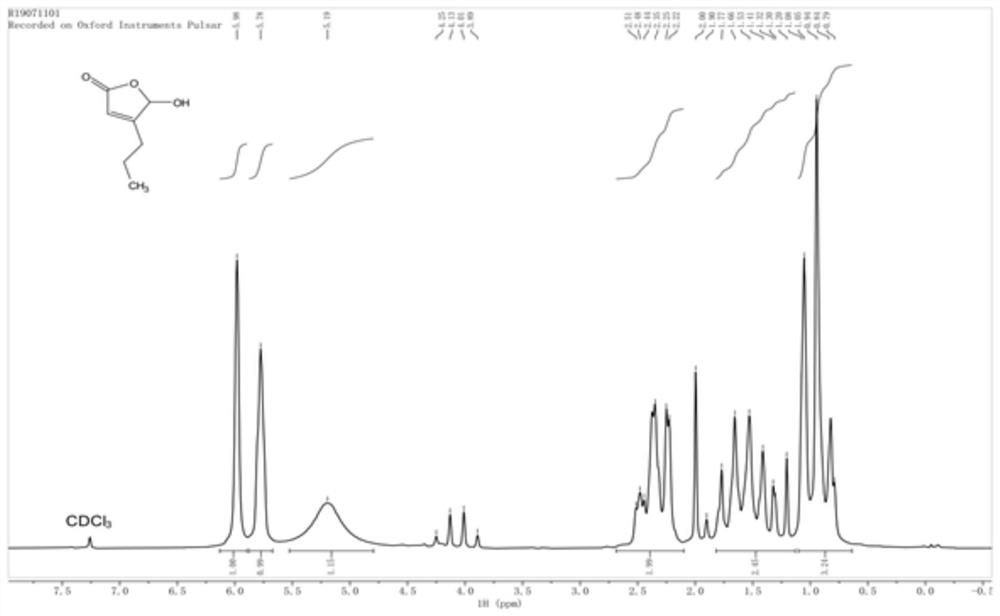
[0054] Preparation of 5-hydroxy-4-n-propyl-2(5H)-furanone
[0055] At room temperature, add 15 mL of distilled water and 8.89 g of morpholine (molecular weight 87.1, 0.102 mol) into a 100 mL three-necked flask, cool to 0-5°C, and slowly add 14.8 g of 50% by volume glyoxylic acid solution (molecular weight 74, 0.1 mol), the temperature was controlled below 15°C during the dropwise addition, and the stirring was continued for 15 minutes after the dropwise addition was completed. Then slowly add 8.61g of valeraldehyde (molecular weight: 86.1, 0.1mol) dropwise at 15-25°C under temperature control. Note: there is no smell of aldehyde in the post-treatment process. The reaction solution was cooled to room temperature, and 12 mL of 37% hydrochloric acid (molecular weight 37.5, 0.14 mol) was added dropwise, and stirring was continued at 23-25° C. for 3 hours after the addition was completed. Subsequently, add 30mL tertiary methyl ether (thin-layer chromatography TLC), separate layers...
Embodiment 2
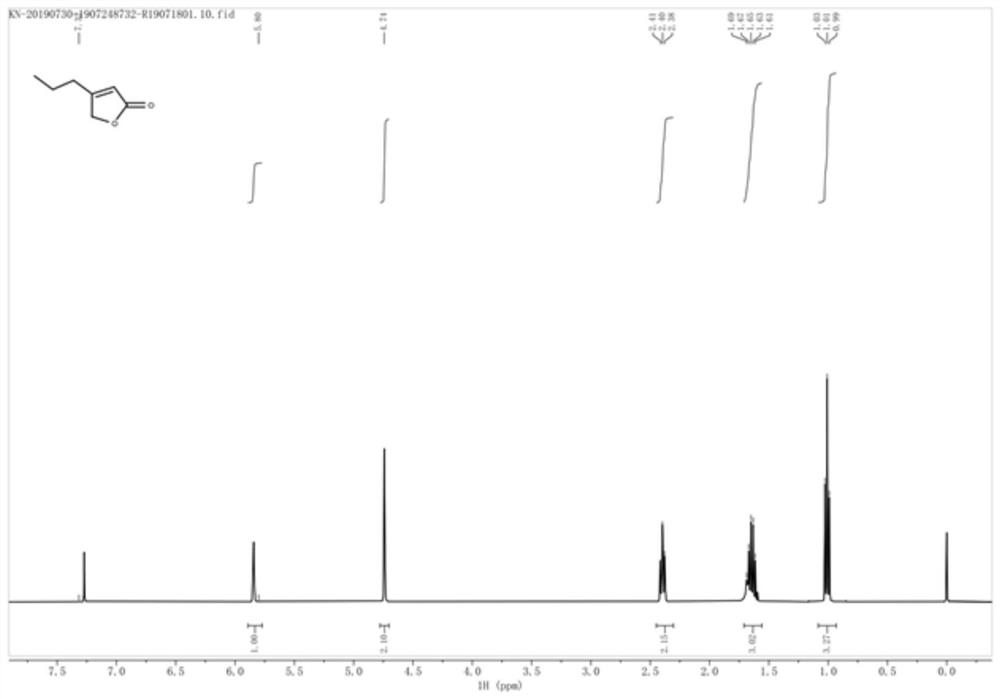
[0057] Preparation of 4-n-propyl-2(5H)-furanone
[0058] Add 50mL of anhydrous methanol and 6.9g of 5-hydroxy-4-n-propyl-2(5H)-furanone (molecular weight 142, 48.6mmol, 1eq) into a 100mL three-necked flask, cool to -5~0°C, and batch After adding 3.9g of sodium borohydride (molecular weight 37.8, 103mmol, 2.1eq), the temperature was raised to 0-10°C (note: gas was generated, exothermic violently and with delay), continued to stir for 30min, and then naturally rose to room temperature, and thin-layer chromatography The progress of the reaction was detected by TLC (developing solvent: n-hexane / ethyl acetate = 3:1). After the raw materials completely disappeared, cool to 0-5°C, slowly add 100mL of hydrochloric acid dropwise, and continue stirring for 5 minutes after the dropwise addition, and 80mL of acetic acid Extract with ethyl ester 3 times (note: the pH of the aqueous phase = 4.0-4.5), combine the organic phases, wash once with 100 mL of 5% sodium carbonate, wash once with sa...
Embodiment 3
[0060] Preparation of recombinant Escherichia coli wet cells
[0061] The expression vector pET-28a that has been digested with two endonucleases Nco I and EcoR I is connected with the nucleotide sequence of the enone reductase, the ketoreductase sequence, and the glucose dehydrogenase gene respectively with T4 ligase ,overnight. Add 1 microliter of the ligation product to the electroporation containing 50 microliters of E. coli electrocompetent cells, and immediately shock on the electroporator, then immediately transfer to ice, and add the broth preheated to 37°C respectively Mix 1mL of agar medium; transfer the mixed medium to 2mL culture tubes, and incubate at 200°C for one hour at 37°C; carry out on a broth agar plate containing 100 microliters per mL of kanamycin Streak culture, 37 ° C incubator overnight culture for 16 hours; the next day, pick a single clone on the inoculation plate and inoculate it into a Erlenmeyer flask containing 15 mL of kanamycin medium, 37 ° C ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com