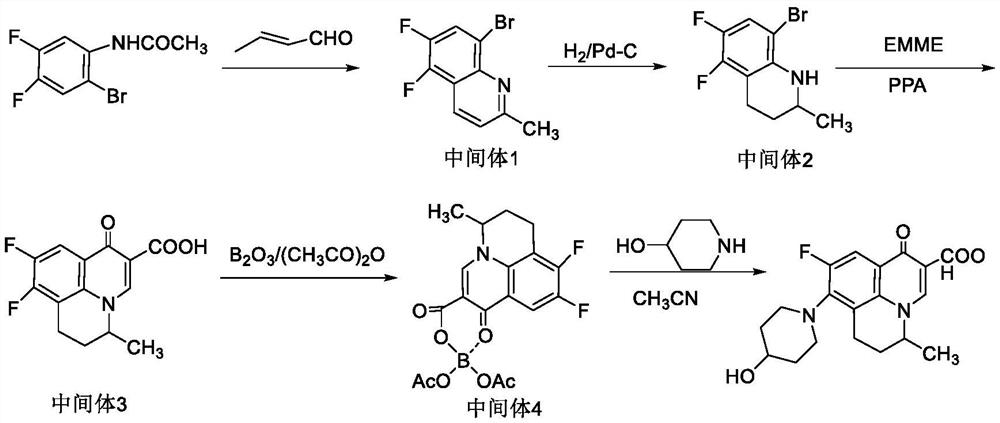
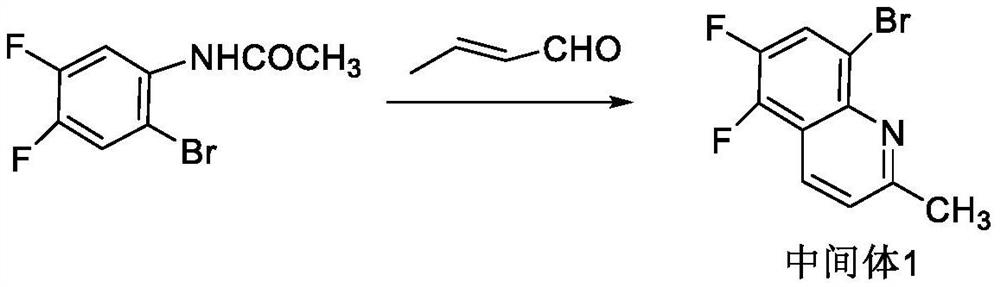
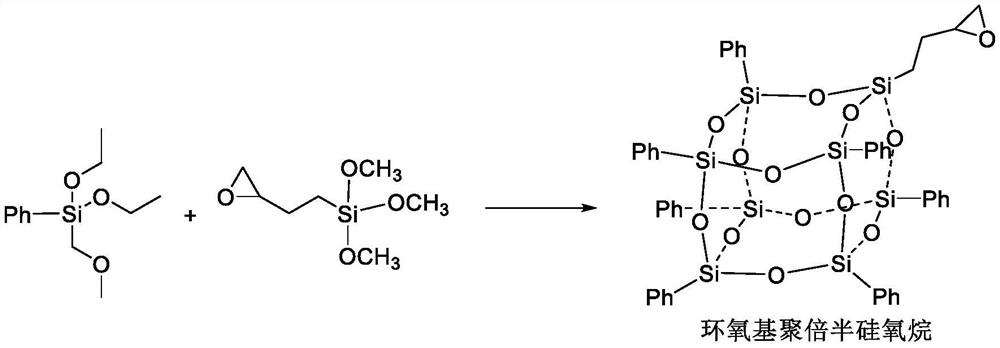
Synthesis process of S-(-)-nadifloxacin chiral intermediate
A chiral intermediate, nafloxacin technology, applied in the directions of silicon organic compounds, organic chemistry, organic chemistry methods, etc., can solve the problems of low compound utilization rate and high cost of chemical resolution methods, and improve chiral selectivity. , the effect of increasing solubility and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Chiral ligands are made by the following steps:
[0034] S1. Add 30 mL of ethanol into a three-necked flask equipped with a magnetic stirrer, a thermometer and a reflux condenser, heat the temperature of the reaction system to 35°C, then add 1.6 mol of deionized water and 10 mL of hydrochloric acid, stir for 25 minutes, and dropwise add 0.75 mol of the mixture of triethoxysilane and epoxybutyltrimethoxysilane, the dropping rate is 1 drop / second, after the addition is complete, the hydrolysis reaction is continued for 3 days, and then the pH of the reaction solution is adjusted to neutral with NaOH aqueous solution. The solvent was removed by distillation under reduced pressure, washed twice with deionized water, dried over anhydrous magnesium sulfate, and filtered to obtain epoxy polysilsesquioxane; containing phenyl triethoxy silane and epoxy butyl trimethoxy The molar ratio of phenyltriethoxysilane to epoxybutyltrimethoxysilane in the mixture of base silane is 6.8:1, ...
Embodiment 2
[0039] Chiral ligands are made by the following steps:
[0040] S1. Add 50 mL of ethanol into a three-necked flask equipped with a magnetic stirrer, a thermometer and a reflux condenser, heat the temperature of the reaction system to 35°C, then add 1.6 mol of deionized water and 12 mL of hydrochloric acid, stir for 25 minutes, and dropwise add 0.8 mol of the mixture of oxybutyltriethoxysilane and epoxybutyltrimethoxysilane, the rate of addition is 1 drop / second, after the addition is complete, the hydrolysis reaction is continued for 3 days, and then the pH of the reaction solution is adjusted to neutral with NaOH aqueous solution. The solvent was removed by distillation under reduced pressure, washed 2-4 times with deionized water, dried over anhydrous magnesium sulfate, and filtered to obtain epoxy polysilsesquioxane; containing phenyltriethoxysilane and epoxybutyl The molar ratio of phenyltriethoxysilane and epoxybutyltrimethoxysilane in the mixture of trimethoxysilane is 7...
Embodiment 3
[0048] Chiral catalysts are made by the following steps:
[0049] Add 0.1 mmol RuCl to the three-necked flask under nitrogen protection 3 ·xH 2 O and 0.21 mmol of the chiral ligand prepared in Example 1, then added 6 mL of DMF that had been treated with dehydration and oxygen removal, stirred at 100°C for 1 h, cooled to room temperature, stirred overnight, vacuum-dried the DMF at 35°C, and removed the residue Dichloromethane was added to the mixture, filtered under nitrogen protection, the filtrate was concentrated in vacuo, n-hexane was added, and filtered under nitrogen protection to obtain a chiral catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com