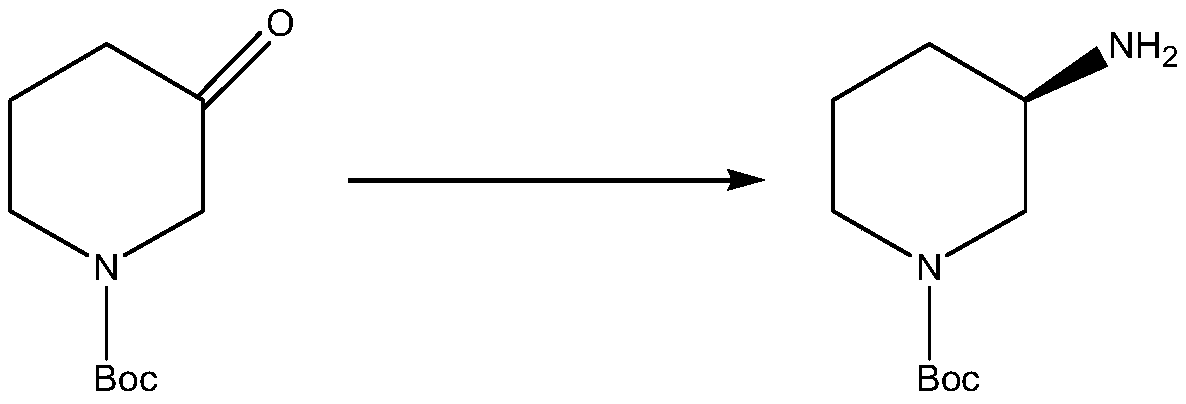
Method for synthesizing (R)-1-tert-butoxycarbonyl-3-aminopiperidine by adopting transaminase catalyst and enzymatic way
A technology of tert-butoxycarbonyl and aminopiperidine, applied in the field of enzyme catalysis, can solve the problems of inability to obtain high optical purity, cumbersome procedures, complicated operations, etc., and achieves the effects of high specificity, broad application prospects and strong catalytic activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] According to this embodiment, the recombinant Escherichia coli expressing the transaminase catalyst is cultivated:
[0049] The synthetic transaminase catalyst gene DNA fragment was double-digested with restriction endonuclease NdeI and AvrII at 37°C for 8 h, purified by agarose gel electrophoresis, and the target fragment (SEQ ID NO. 1); then, under the action of T4 DNA ligase, the target fragment was ligated with the plasmid pACYC-Duet-B that was digested by NdeI and EcoRI overnight at 25°C to obtain a recombinant expression plasmid; the recombinant expression plasmid was transformed into In Escherichia coli competent cells, the transformation conditions are: 45°C, heat shock for 90 seconds, screen the positive recombinants on a resistance plate containing chloramphenicol, pick a single clone, culture the recombinant bacteria, and wait for the plasmid After amplification, the plasmid was extracted and retransformed into competent cells. The transformation solution was...
Embodiment 2
[0052] Transaminase Catalyst Activity and Chiral Verification
[0053] The catalytic system is as follows (total volume 5mL): 0.25g (50g / L) recombinant Escherichia coli wet cells expressing transaminase catalyst, 0.1M TEOA buffer solution (pH8), 2mM pyridoxal phosphate, 100g / L N-tert-butoxy Carbonyl-3-piperidone, 0.5M isopropylamine (adjust pH to 8 with hydrochloric acid); reaction conditions: temperature 30°C, 400rpm magnetic stirring, reaction for 24 hours. After the reaction was completed, it was inactivated with acetonitrile at a ratio of 1:1, and a sample was taken to detect the formation of the product by HPLC. It was calculated that the conversion rate was 25% after 24 hours of reaction, and 12.5 g of the product was produced. After detection, the product is the R enantiomer, and the ee value is above 99.77%.
Embodiment 3
[0055] Substrate Concentration Optimization
[0056] The catalytic system is as follows (total volume: 5 mL): 0.25 g (50 g / L) of recombinant E. coli wet cells expressing transaminase catalyst, 0.1 M TEOA buffer solution (pH8), 2 mM pyridoxal phosphate, 4-100 g / L N-tert Butoxycarbonyl-3-piperidone, 0.5M isopropylamine (adjust pH to 8 with hydrochloric acid); reaction conditions: temperature 30°C, 400rpm magnetic stirring, reaction for 24 hours.
[0057] After the reaction was finished, inactivate with acetonitrile 1:1, sample HPLC to detect the generation of the product, the results are shown in Table 2 below:
[0058] Table 2 The amount of product generation corresponding to different substrate concentrations
[0059]
[0060]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com