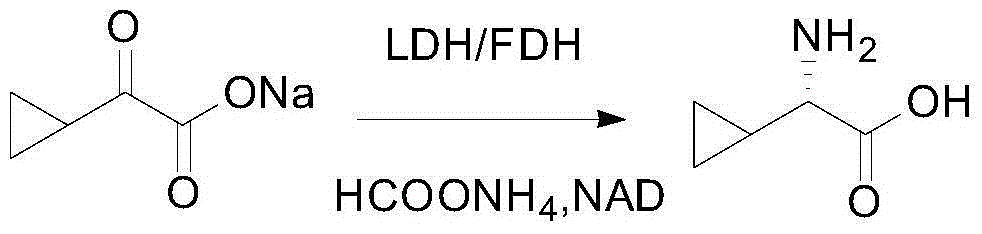Synthetic method of l-cyclic alkyl amino acid and pharmaceutical composition having same
The technology of an alkyl amino acid and its synthesis method is applied in the field of synthesis method and pharmaceutical composition with it, which can solve the problems of high price, long synthesis route, and a large amount of organic solvents, and achieve mild reaction conditions, high chiral selectivity, The effect of high conversion rate of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] In a typical embodiment of the present invention, a method for synthesizing L-cyclic alkyl amino acids is provided, which includes: step A, preparing a cyclic alkyl keto acid having structural formula (I) or structural formula (II) or Cyclic alkyl keto acid salt; step B, combining cyclic alkyl keto acid or cyclic alkyl keto acid salt with ammonium formate, leucine dehydrogenase, formate dehydrogenase and coenzyme NAD + Mix and carry out reductive amination reaction to produce L-cyclic alkyl amino acid, wherein the structural formula (I) is n 1 ≥1, m 1 ≥0, M 1 Is H or a monovalent cation; structural formula (Ⅱ) is n 2 ≥0, m 2 ≥0, M 2 It is H or a monovalent cation; the amino acid sequence of leucine dehydrogenase is SEQ ID No. 1.
[0030] The above-mentioned synthetic method utilizes the specific leucine dehydrogenase and formate dehydrogenase with the amino acid sequence of SEQ ID No. 1 and the coenzyme NAD + Cooperate to make cyclic alkyl keto acid undergo reductive amina...
Embodiment 1
[0058] Synthesis of L-cyclopropylglycine
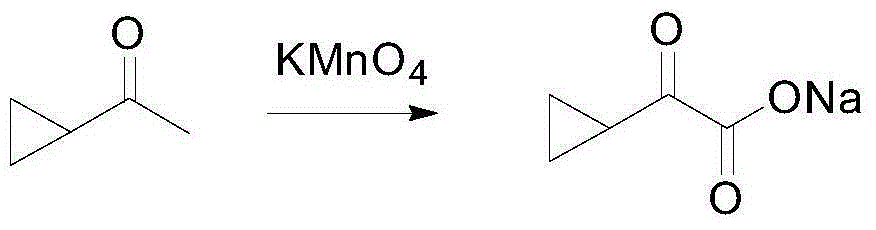
[0059] 1) At room temperature, add 500g of cyclopropyl methyl ketone, 15.6g of NaOH, and 8.7L of water to a 20L four-necked flask to form a mixed system. After the mixed system is heated to 45~55℃, add 8.7L of water to the mixed system. 1864.8gKMnO 4 Aqueous solution, undissolved KMnO 4 Then add it to the mixed system in batches. After about 10 hours, the addition is complete. The reaction tracking raw materials are completely reacted. The internal standard yield is 57.9%. Add 10% NaHSO to the mixed system after the reaction is completed. 3 Destroy unreacted KMnO 4 ; Suction filtration, the filtrate is concentrated to 70% and directly used in the next step. 1HNMR (500MHz, CD 3 Cl): δ 1.29 (m, 3H), 1.06 (m, 1H), 0.95 (m, 1H).
[0060]
[0061] 2) At room temperature, add 1,627 g of the 14.5% sodium cyclopropyl oxoacetate aqueous solution, 193.3 g of ammonium formate, and 2350 mL of leucine dehydrogenase with an enzyme specific activity of 6...
Embodiment 2
[0064] Synthesis of L-cyclobutylglycine
[0065] 1) Add 15.13g of magnesium chips, 160ml of tetrahydrofuran, and 2 iodine granules to a 1L four-necked flask, and then add 32ml of tetrahydrofuran solution with 8g of bromocyclobutane to it, and heat the four-necked flask to make the bromocyclobutane in the system The alkane format reagent is initiated; then the four-neck flask is cooled to 40°C, and 288ml of tetrahydrofuran solution with 72g of bromocyclobutane is added dropwise to it. After about 2.5 hours, the bromocyclobutane reacts according to the following reaction formula; After incubating at 40-50°C for about 1 hour, the Grignard reagent is obtained, and the temperature is reduced to room temperature under nitrogen protection for later use.
[0066]
[0067] 2) Add 160ml of tetrahydrofuran solution with 112.6g of diethyl oxalate to a 1L four-necked flask, cool the four-necked flask to below -50°C with liquid nitrogen and ethanol; A good 0.593mol format reagent is pressed int...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com