Synthetic method of dehydroprogesterone
A technique for dehydroprogesterone and its synthetic method, which is applied in the fields of steroids and organic chemistry, can solve problems such as low yield, limited technology, and difficult scale-up of production, and achieve high product yield, short synthetic steps, The effect of less isomer impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
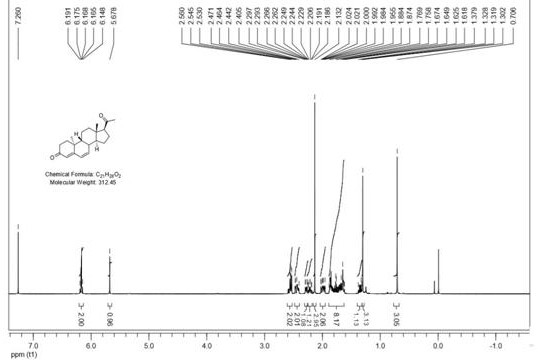
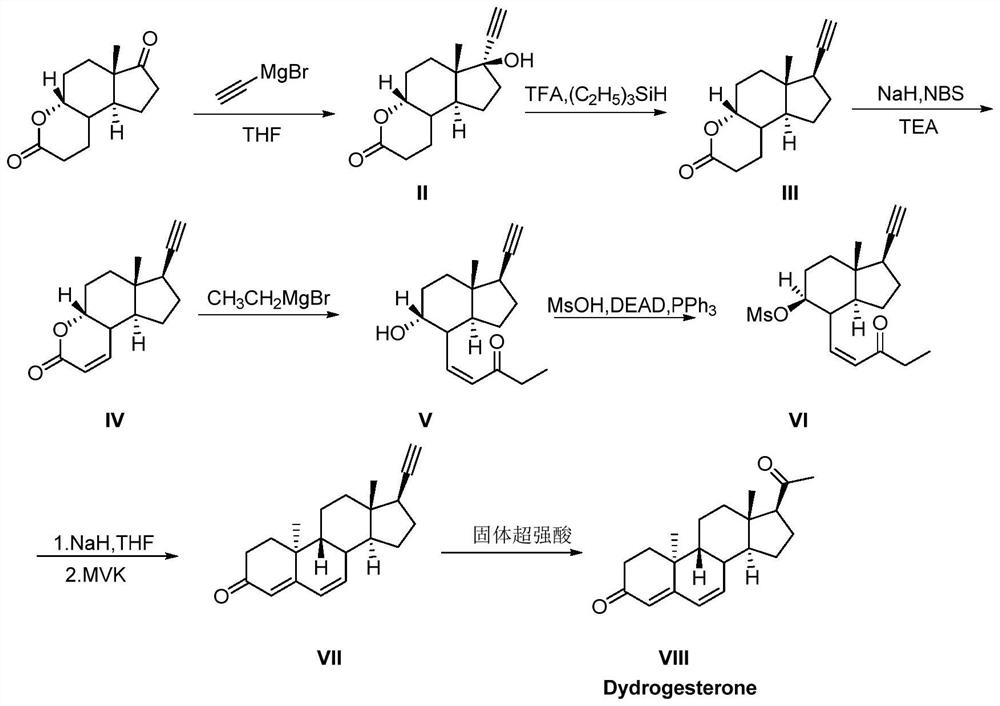
[0043] provided as Figure 1-4 The synthetic method of dehydroprogesterone shown in embodiment 1, concrete steps are as follows:
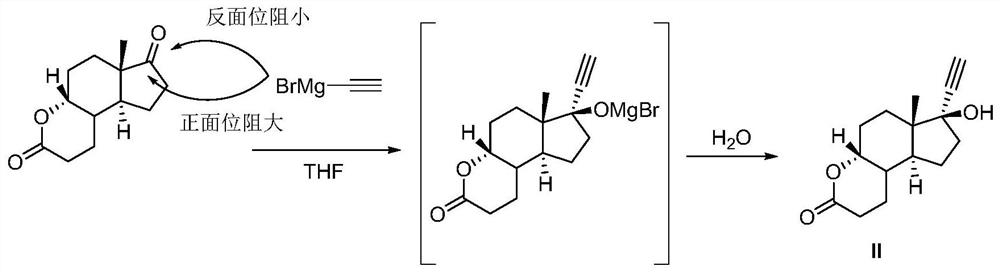
[0044] S100: Add ring A degradation products (200g, 0.9mol), THF (2000mL, 10v / w) into a 5L four-necked flask equipped with a dropping funnel, a thermometer and mechanical stirring, respectively, under nitrogen protection, then start stirring and cool down to ≤-50°C, then add 0.5M ethynylmagnesium bromide tetrahydrofuran solution (1.9L, 0.95mol, 1.05eq) dropwise to the above reaction system, control the reaction temperature not to exceed -45°C, after the addition is complete, then stir at constant temperature 30min. After the reaction is monitored by TLC, the above reaction solution is poured into a saturated ammonium chloride solution to quench, and the water phase is extracted once again after standing for stratification, and the combined organic phase is washed with a saturated sodium chloride solution, dried over anhydrous sodium sulfate, and f...
Embodiment 2
[0063] Based on Example 1, in this example, ethynylmagnesium chloride is used as the Grignard reagent at S100 to react, and other conditions remain unchanged, and the yield of compound II is 85%, and the reaction conditions of other S200-S700 remain unchanged, and its The yield of each step of S200-S700 does not change. Then the total yield of pure dehydroprogesterone is 29.6%.
Embodiment 3
[0065] Based on Example 1, in this example, DIPEA is used instead of TEA at S300, and other conditions remain unchanged, and the yield of compound IV is 60%. The reaction conditions of other S100-S200 and S400-S700 remain unchanged, and the above steps yield remains unchanged. Then the total yield of pure dehydroprogesterone is 29.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com