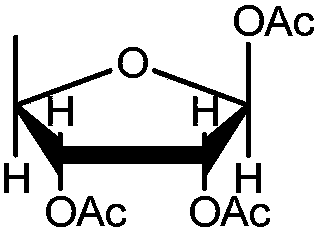
Preparation method of high-purity capecitabine key intermediate
A technology of Chinese formula and compound, which is applied in the field of medicine and drug synthesis, can solve the problems of high cost of raw materials, difficulty in recycling, and expensive pyridine, etc., and achieve the effects of small α-isomer content, mild reaction conditions, and high chiral selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
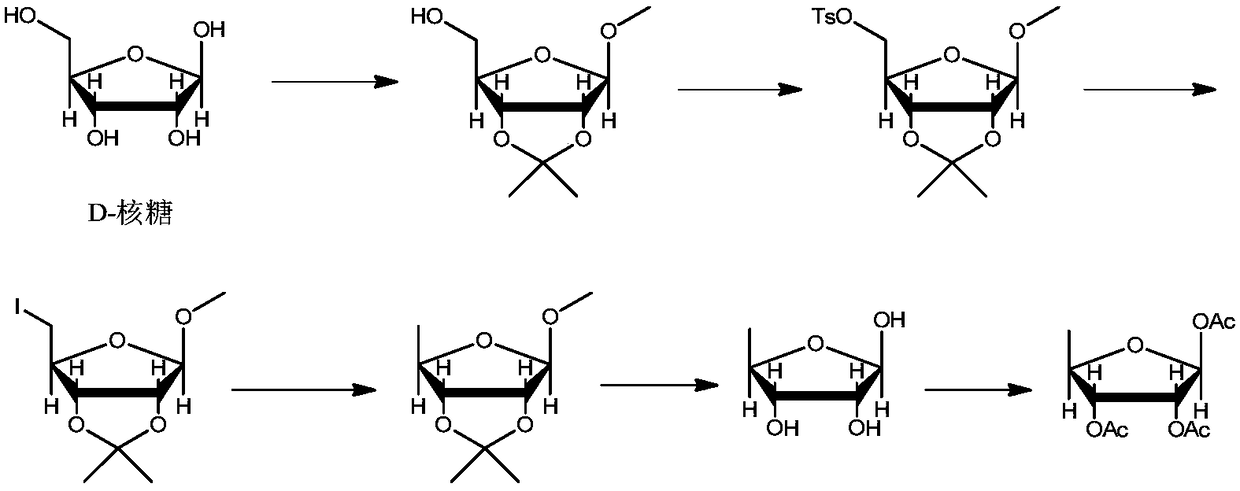
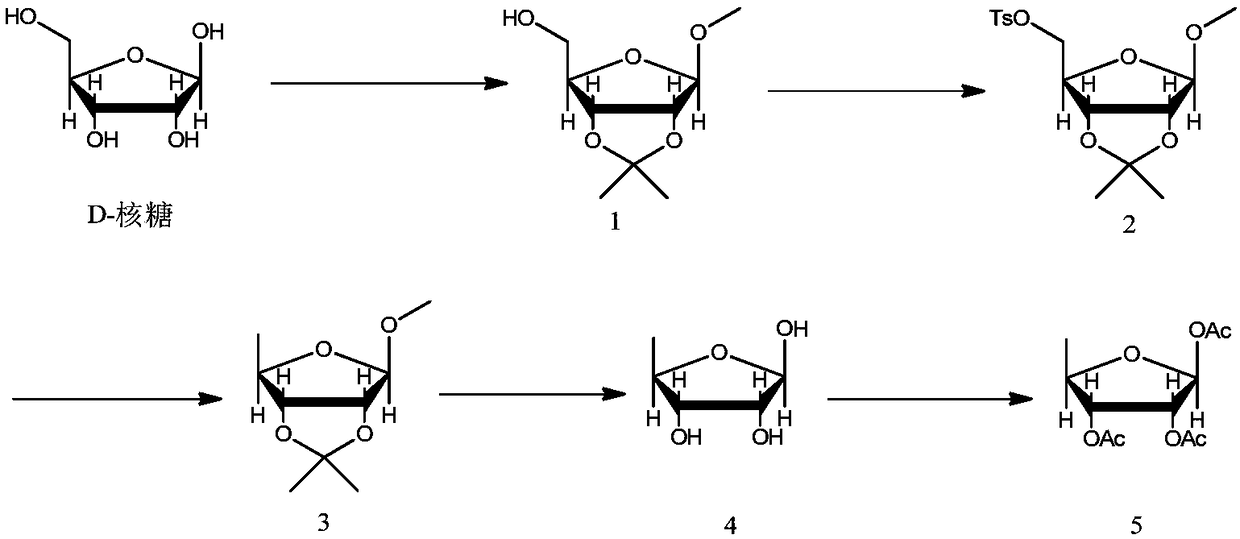
[0043] Example 1: Preparation of compound of formula 1
[0044]
[0045] Add anhydrous methanol (2.4kg), acetone (0.8kg) to the dry three-necked flask, add concentrated sulfuric acid (164g) dropwise in an ice bath, add D-ribose (500g), control the temperature at 10-20℃, keep warm and stir for 20~ 30 hours. After the completion of the reaction monitored by TLC, the reaction liquid was added dropwise to a solution of sodium hydroxide (133 g) in methanol (666 g), and the methanol and acetone were removed by concentration. Add water (2.5kg) and toluene (2.5kg) to the concentrate, stir and extract, separate liquids, extract the aqueous phase toluene (500g) twice, combine the organic phases, wash once with saturated brine (1.5kg), and use the organic phase directly Next reaction.
Embodiment 2
[0046] Example 2: Preparation of the compound of formula 2
[0047]
[0048] The toluene solution of the compound of formula 1 obtained in Example 1, 4-dimethylaminopyridine (0.3g), and sodium hydroxide (195.0g) were sequentially added to the four-neck flask, the temperature was controlled at 5-15°C, and p-toluenesulfonate was added dropwise A solution of acid chloride (560.0g) in toluene (600g). After the dripping is completed, the temperature is controlled and reacted for 1 to 5 hours. After the completion of the reaction is monitored by TLC, water (2kg) is added and the liquids are separated. The water phase is extracted once with toluene (1.0kg). The organic phases were combined, washed once with saturated brine (2kg), the organic layer was concentrated and ethanol (2.5kg) was added to raise the temperature to 60-75°C, and after dissolution, the temperature was lowered to 0-10°C. After filtration, the filter cake was rinsed once with ethanol, and dried by blowing to obtain 95...
Embodiment 3
[0049] Example 3: Preparation of compound of formula 3
[0050]
[0051] The compound of formula 2 (317g), N,N-dimethylacetamide (915g), potassium borohydride (57.1g) were added to the reaction flask in sequence, the temperature was raised to 75~90℃, and the reaction was carried out at this temperature for 15~ After 25 hours, TLC monitors the completion of the reaction, cool to room temperature, add water (2.3kg) to quench the reaction, add n-hexane (1.2kg) for extraction three times, combine the organic layers, and concentrate to dryness to obtain 161.0g of a colorless liquid compound of formula 3. The yield was 96.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com