Transaminase catalyst and method for enzymatic synthesis of (R)-1-naphthylethylamine
A catalyst and transaminase technology, applied in biochemical equipment and methods, botany equipment and methods, microbial-based methods, etc., can solve the problems of low chiral resolution yield, high industrial production costs, and potential safety hazards , to achieve the effect of avoiding chemical resolution steps, easy to obtain, and stable at the end of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
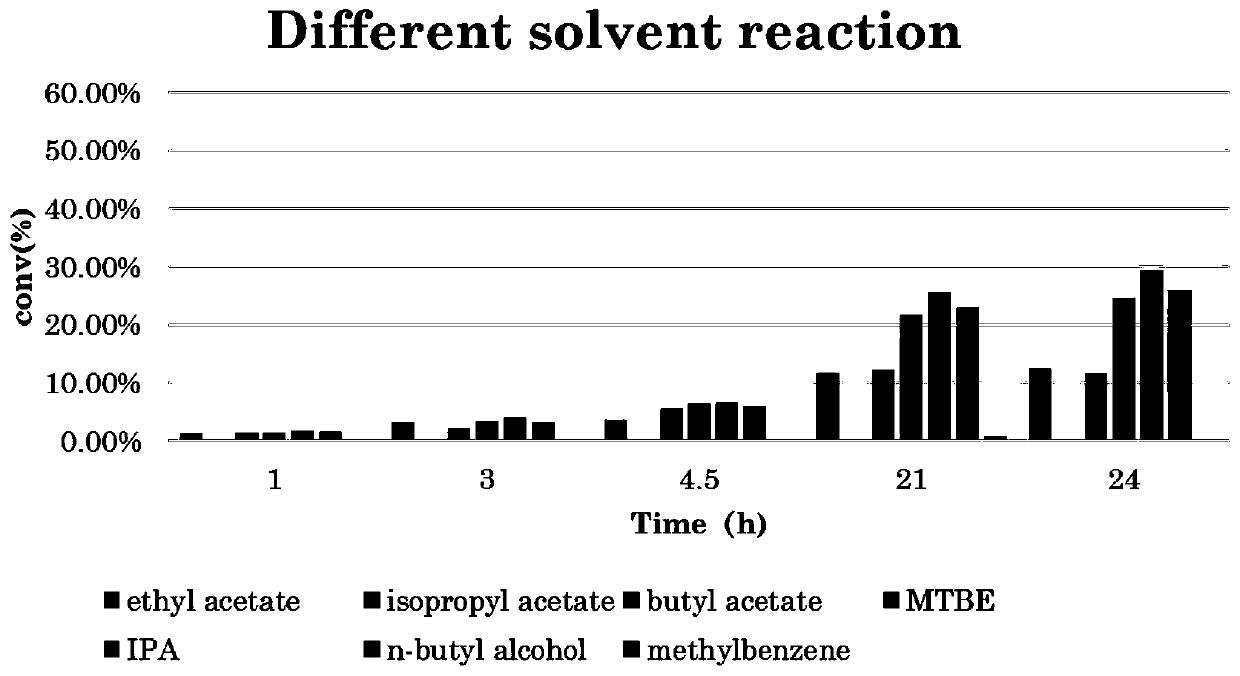
[0062] This embodiment aims to compare the impact of different solvents on the reaction conversion rate, and the specific experimental conditions include:
[0063] The total volume of the reaction system: 5ml;
[0064] 1-naphthylethanone (substrate) concentration: 100g / L;
[0065] Recombinant Escherichia coli wet cell concentration: 100g / L;
[0066] Isopropylamine concentration: 2M;
[0067] PLP concentration: 2mM;
[0068] The solvents are: ethyl acetate, isopropyl acetate, butyl acetate, isopropanol, n-butanol, methyl tert-butyl ether, toluene;
[0069] Reaction temperature: 30°C;
[0070] Reaction time: 1h, 3h, 4h, 5h, 21h, 24h;
[0071] The specific reaction results are as follows figure 1 As shown, it can be seen that when isopropanol (IPA) is used as the reaction solvent, the enzyme activity is the best.
Embodiment 2
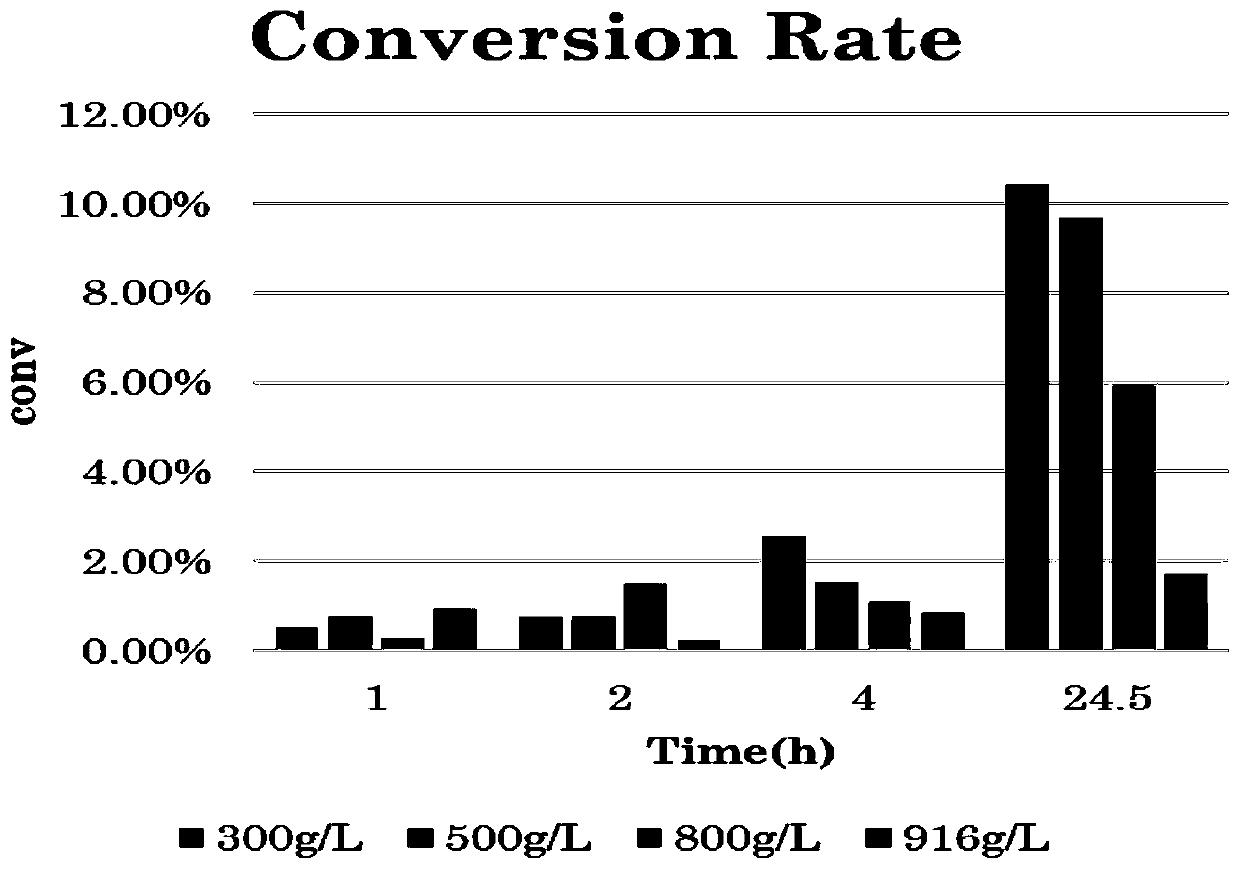
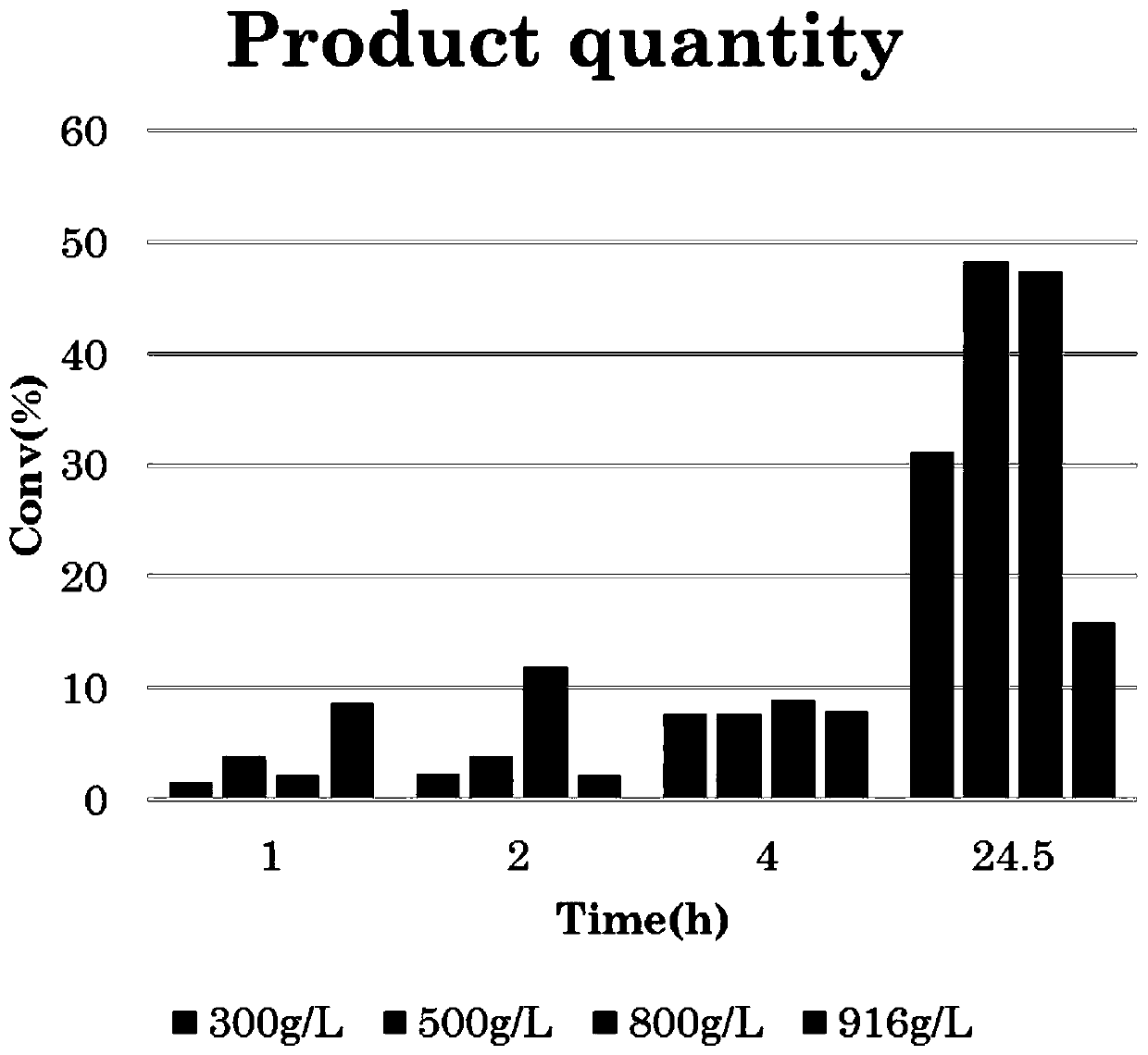
[0073] This embodiment is intended to implement the substrate gradient experiment, and the specific experimental conditions include:
[0074] The total volume of the reaction system: 5ml;
[0075] 1-naphthylethanone (substrate) concentration: 300-916g / L (full substrate);
[0076] Recombinant Escherichia coli wet cell concentration: 100g / L;
[0077] Isopropylamine concentration: 2M;
[0078] PLP concentration: 2mM;
[0079] Isopropanol concentration: 37%;
[0080] Reaction temperature: 30°C;
[0081] Reaction time: 1h, 2h, 4h, 24.5h;
[0082] The specific reaction results are as follows figure 2 with 3 As shown, when the visible substrate concentration is 500g / L, the 24.5h substrate accumulation is the largest.
Embodiment 3
[0084] 1) In a three-necked flask, add 90 g of recombinant Escherichia coli wet cells expressing the transaminase catalyst, 450 g of 1-naphthyloethanone, place in a water bath at 30° C., and stir mechanically;
[0085] 2) Add 270ml isopropanol, 144ml isopropylamine and 9ml PLP aqueous solution while stirring;
[0086] 3) stirring and reacting at a speed of 200r / min for 48 hours;
[0087] 4) After the reaction, distill under reduced pressure at 55°C to remove isopropanol and isopropylamine;
[0088] 5) Filtrate, add 250ml of 3M hydrochloric acid aqueous solution to the filtrate, cool down and crystallize to obtain 140g of solid naphthylethylamine hydrochloride, then add sodium hydroxide to dissociate, extract with 100ml of dichloromethane, leave to separate layers, take the organic phase, and distill off the solvent to obtain The target product (R)-1-naphthylethylamine was 108g, and the ee value of the target product was >99% after detection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com