Synthetic method of L-heterocyclic amino acid and pharmaceutical composition with L-heterocyclic amino acid
A technology of a heterocyclic amino acid and a synthesis method, which is applied in the field of synthesis methods and pharmaceutical compositions having the same, can solve the problems of high price, long synthesis routes, a large amount of organic solvents, etc., and achieves mild reaction conditions, simplified synthesis techniques, and chirality. Highly selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
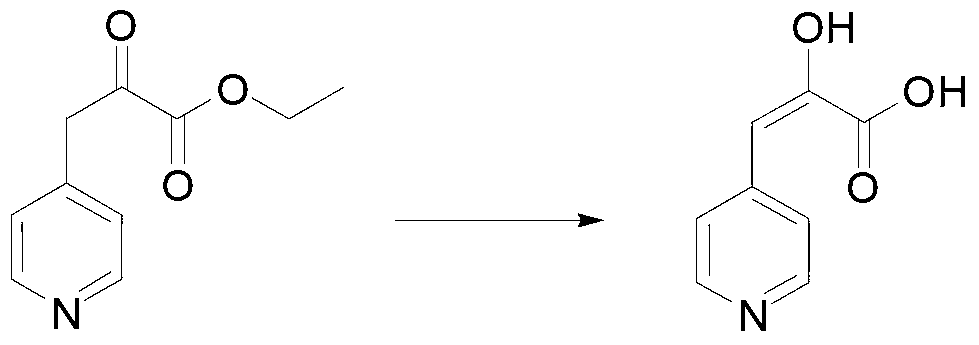
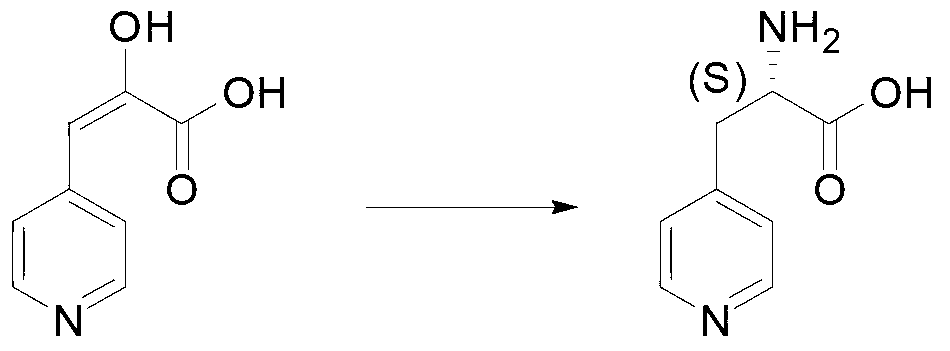
[0020] In a typical embodiment of the present invention, a method for synthesizing an L-heterocyclic amino acid is provided, comprising: step A, preparing a heterocyclic keto acid, wherein the heterocycle in the heterocyclic keto acid is selected from five-membered Any of heterocycle, six-membered monoheterocycle, seven-membered monoheterocycle, five-membered alkyl-substituted monoheterocycle, six-membered alkyl-substituted monoheterocycle, and seven-membered alkyl-substituted monoheterocycle, heterocyclic ketone The structural formula of the ketoacid group in an acid is and be located at any carbon position of the heterocycle; step B, combine the heterocyclic ketoacid with ammonium formate, phenylalanine dehydrogenase, formate dehydrogenase and coenzyme NAD + Mixing and performing reductive amination reaction to generate L-heterocyclic amino acid, wherein the amino acid sequence of phenylalanine dehydrogenase is SEQ ID No.1.
[0021] The above synthesis method utilizes the ...
Embodiment 1
[0040] Synthesis of L-4-pyridylalanine
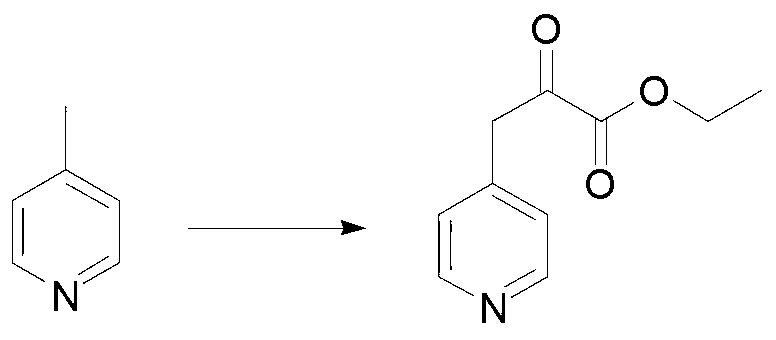
[0041] 1) Add 904.2g, 1.5eq potassium tert-butoxide, 2L tetrahydrofuran and 500g 4-picoline to the four-necked flask, stir at room temperature for 2.5h, add 941.1g diethyl oxalate dropwise, and stir overnight at room temperature until the reaction is complete , the specific reaction is as follows. Temporary storage of the system goes directly to the second step.
[0042]
[0043] 2) Put the above system into the bottle, then add 1L methanol, 2L H 2 0, 783.6g potassium tert-butoxide, insulation reaction to no raw material, concrete reaction is as follows. Then concentrate, and lower the concentrated system to room temperature, adjust the pH value between 2 and 3 with 6mol / L hydrochloric acid, add 3 times the volume of water to it for dilution, and filter with suction, and the filtrate drops to 0 to 5°C. , to obtain 640g solid, two-step yield 72.2%. 1 H NMR (400 MHz, DMSO): δ 8.45 (d, 2H), 8.03 (d, 2H), 7.55 (d, 1H).
[0044]
...
Embodiment 2
[0048] Synthesis of L-2-pyridylalanine
[0049] 1) Add 800mL tetrahydrofuran and 152g (1.4eg) redistilled diisopropylamine to a 2L four-necked bottle, stir and cool down to -50~-40℃, add dropwise 590mL n-butyllithium (2.55 N, 1.4eq), then stirred for 0.5h, continued to cool down to -80~-70℃, added 100g of 2-picoline dropwise at -80~-70℃, kept it warm for 2h, followed TLC until the raw materials disappeared, and obtained System A;
[0050] Add 200mL tetrahydrofuran and 172g diethyl oxalate to a 3L four-neck flask, stir evenly, then cool down to -80~-70°C to obtain System B;
[0051] Press system A into system B at -80~-70°C, stir for 1 hour and follow TLC until the reaction is complete, return to -60°C, control the system temperature below -20°C, and use 2mol / L hydrochloric acid to adjust the system pH Adjust the temperature between 5 and 6, raise the temperature of the system to room temperature, and extract the aqueous phase with 300mL ethyl acetate three times after liquid ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
| chiral purity | aaaaa | aaaaa |
| chiral purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com