Preparation method of moxifloxacin intermediate
A compound and reaction technology, applied in the field of preparation of moxifloxacin intermediates, can solve problems such as low yield, complex process flow, and low chiral selection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073]
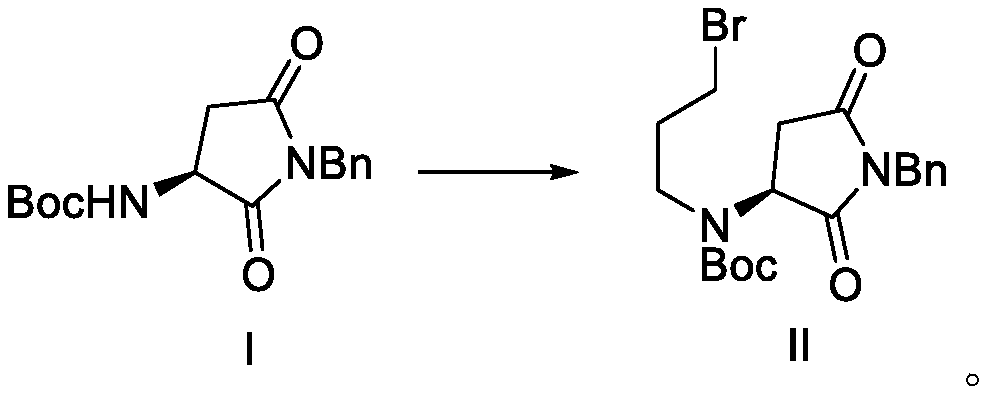
[0074] The raw material N-Boc-aspartic acid benzylimide can be synthesized according to the literature Tetrahedron Letters 2004,3603.
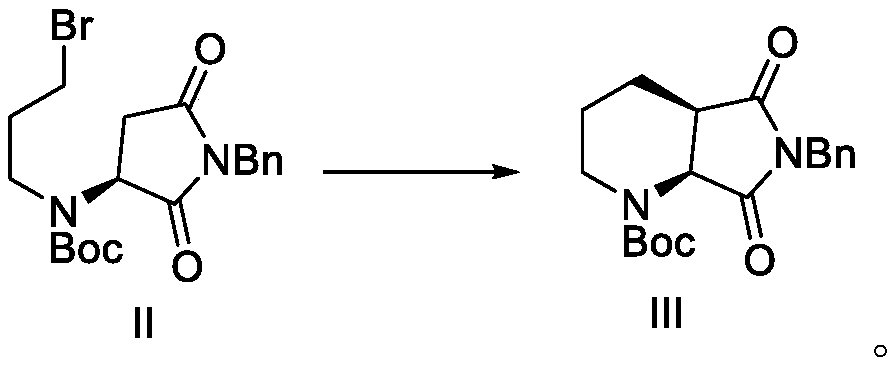
[0075] Dissolve 304 grams of raw material N-Boc-aspartic acid benzylimide in 2 liters of dimethylformamide, add 40 grams of sodium hydrogen in batches at room temperature, after the addition, stir for one hour, and slowly add dibromide dropwise 404 g of propane, after adding, stir until the TLC detection reaction is complete, add water to quench the reaction, extract with dichloromethane, dry and concentrate to obtain 350 g of an intermediate (compound shown in formula II) with a yield of 82.3% and a content of more than 97%.
[0076] 1 H NMR(400MHz, CDCl 3 )δ:7.40-7.28(5H,m), 4.90-4.83(1H,m), 4.80(2H,s), 3.52(2H,t,J=6.5Hz), 3.09(1H,dd,J=3.5, 8.9Hz), 2.98 (2H, t, J = 6.5 Hz), 2.79 (1H, dd, J = 3.5, 8.9 Hz), 2.10-2.00 (2H, m), 1.45 (9H, s).
[0077] Dissolve 350 g of the intermediate obtained in the previous step in 1400 mL of anhydrous tetr...
Embodiment 2
[0086] Dissolve 304 kilograms of N-Boc-aspartic acid benzylimide in 2000 liters of dimethylformamide, add 40 kilograms of sodium hydrogen in batches, after the addition, stir for one hour, slowly add dibromopropane 404 dropwise After the addition, stir until the reaction is over, add water to quench the reaction, extract with dichloromethane, dry and concentrate to obtain 350 kg of intermediate, with a yield of 82.3%.
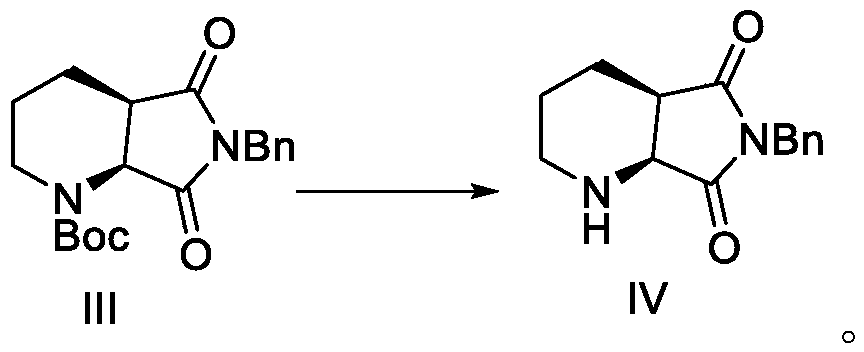
[0087] Dissolve 350 kg of the intermediate in 1400 liters of anhydrous tetrahydrofuran, cool to about -70 degrees, dropwise add 420 liters (2M) of a tetrahydrofuran solution of lithium diisopropylamide, and slowly warm up to room temperature after the addition. After the reaction was completed, water was added to quench the reaction, THF was concentrated, and the residue was extracted with water and ethyl acetate, dried and concentrated to obtain 260 kg of the ring-closing product, the yield: 91.8%.
[0088] Heat 260 kg of the ring-closure product with 1000 liters o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com