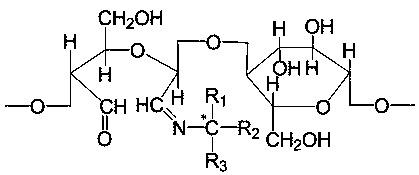
A kind of cellulose chiral derivative and its preparation method and use
A technology of cellulose and derivatives, which is applied in the field of chiral cellulose derivatives and their preparation, can solve the problems of unsuitable chiral stationary phase, poor solubility, non-rigidity, etc., and achieve chiral separation, effect improvement, chiral Effect of separation function improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Take 10cm×30cm filter paper and 10g of cellulose powder and soak them in 100mL 5% NaOH solution for 2h respectively. The treated filter paper is washed until neutral, and dried; the treated cellulose powder is filtered, washed until neutral, and dried.
Embodiment 2
[0025] The filter paper treated in Example 1 was placed in a reactor, and a 2wt% sodium periodate solution (containing periodic acid and periodate aqueous solution) was added at pH=5, and the reaction was carried out in a water bath at 35° C. under light-shielding conditions. After the reaction, wash with ethanol and pure water successively until no IO can be detected. 4 - , air-dried naturally to obtain an oxidized filter paper with an aldehyde content of 29.76%.
Embodiment 3
[0027] The filter paper treated in Example 1 was placed in a reactor, and a 10wt% sodium periodate solution (containing periodic acid and periodate aqueous solution) was added at pH=2, and reacted in a water bath at 45°C for 10 After the reaction, wash with ethanol and pure water successively until no IO can be detected. 4 - , air-dried naturally to obtain an oxidized filter paper with an aldehyde content of 85.06%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com