Synthesis process of trans-menthyl-2, 8-diene-1-ol
A synthetic technique, a menthyl-based technique, which is applied in the field of synthetic technique of trans-menthol-2,8-dien-1-ol, can solve problems such as not seen, and achieve the effect of improving chiral purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A kind of synthetic technique of trans-menthyl-2,8-dien-1-ol, it comprises the steps:
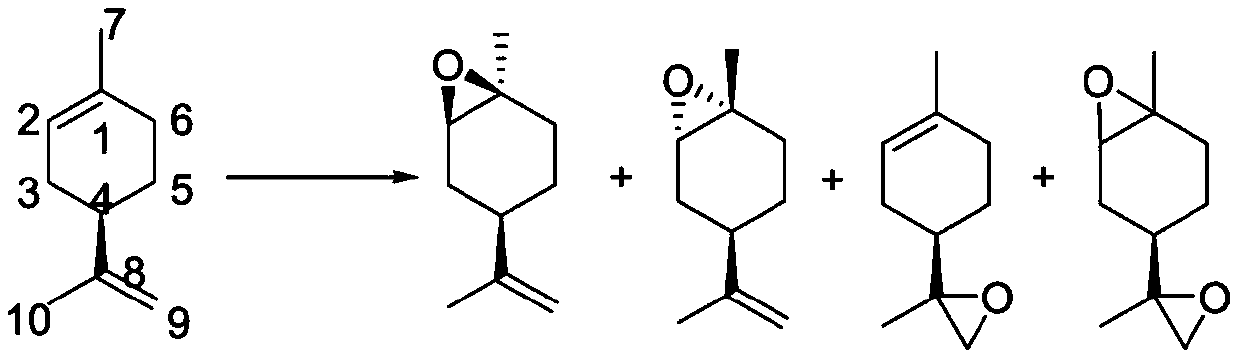
[0041] (1) Add Humicolasp. lipase 48g to the mixed system of (+)-limonene 1.2Kg, purified water 4L and myristic acid 205g, and then slowly pump in 1.1L of 30% hydrogen peroxide at 23°C to control The pumping time is 16 hours. After the pumping, continue to react for 2 hours to stop the reaction, add a 16.7% sodium sulfite aqueous solution to quench, extract with methyl tert-butyl ether, separate liquids twice, combine the obtained organic phases, and sequentially use saturated Sodium bicarbonate aqueous solution and washing with water, and finally the organic phase after washing with water was distilled under reduced pressure to obtain 761 g of trans-1,2-epoxylimonene (purity 91.4%, yield 57%; cis-1,2-epoxylimonene content 1.6 %).
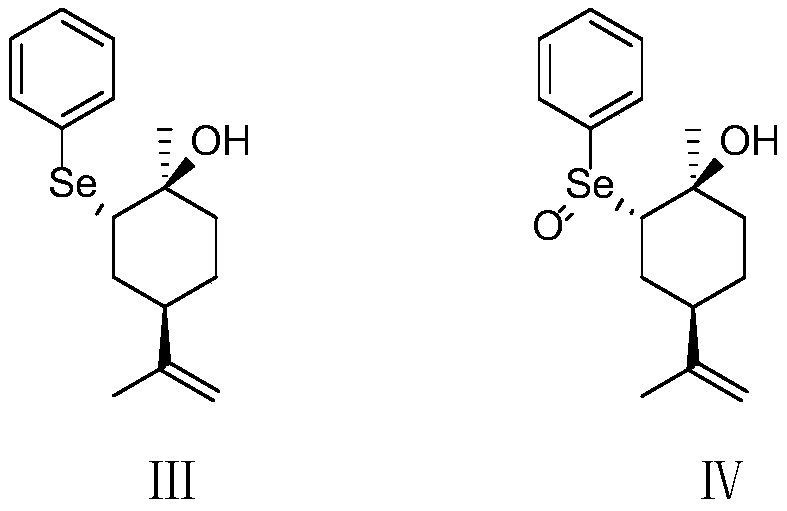
[0042] (2) Under the protection of nitrogen, mix 843g of diphenyl diselenide and 7.6L of absolute ethanol, the temperature drops to 8°C, add 234g of sod...
Embodiment 2
[0047] A kind of synthetic technique of trans-menthyl-2,8-dien-1-ol, its difference with embodiment 1 is:
[0048] (1) Add Humicolasp. lipase 600g to the mixed system of (+)-limonene 1.2Kg, purified water 4L and myristic acid 205g, then slowly pump in 1.1L of 30% hydrogen peroxide at 23°C to control The pumping time is 16 hours. After the pumping, continue to react for 2 hours to stop the reaction, add a 16.7% sodium sulfite aqueous solution to quench, extract with methyl tert-butyl ether, separate liquids twice, combine the obtained organic phases, and sequentially use saturated Sodium bicarbonate aqueous solution and water washing, finally obtain 745g trans 1,2-epoxy limonene (purity 90.1%, yield 56%; cis 1,2-epoxy limonene 1.8% );
[0049] (3) Finally, 501 g of trans-menthyl-2,8-dien-1-ol was obtained (purity 95.0%, ee% 99.0%,
[0050] de% 99.1%).
[0051] The overall yield of the reaction from compound 2 (1,2-epoxylimonene) to compound 5 (trans-menthyl-2,8-dien-1-ol) wa...
Embodiment 3
[0054] A kind of synthetic technique of trans-menthyl-2,8-dien-1-ol, its difference with embodiment 1 is:
[0055] (1) Add Humicolasp. lipase 48g to the mixed system of (+)-limonene 1.2Kg, purified water 4L and myristic acid 205g, and then slowly pump in 2.2L of 30% hydrogen peroxide at 23°C to control The pumping time is 24 hours. After the pumping, continue to react for 2 hours to stop the reaction, add 16.7% sodium sulfite aqueous solution to quench, extract with methyl tert-butyl ether, separate liquid twice, combine the obtained organic phases, and sequentially use saturated Sodium bicarbonate aqueous solution and water washing, finally obtain 776g trans-1,2-epoxylimonene (purity 89.2%, yield 54%; cis-1,2-epoxylimonene 2.3% );
[0056] (3) Finally, 516 g of trans-menthyl-2,8-dien-1-ol was obtained (purity 97.0%, ee% 98.4%, de% 98.9%).
[0057] The overall yield of the reaction from compound 2 (1,2-epoxylimonene) to compound 5 (trans-menthyl-2,8-dien-1-ol) was 65%.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com