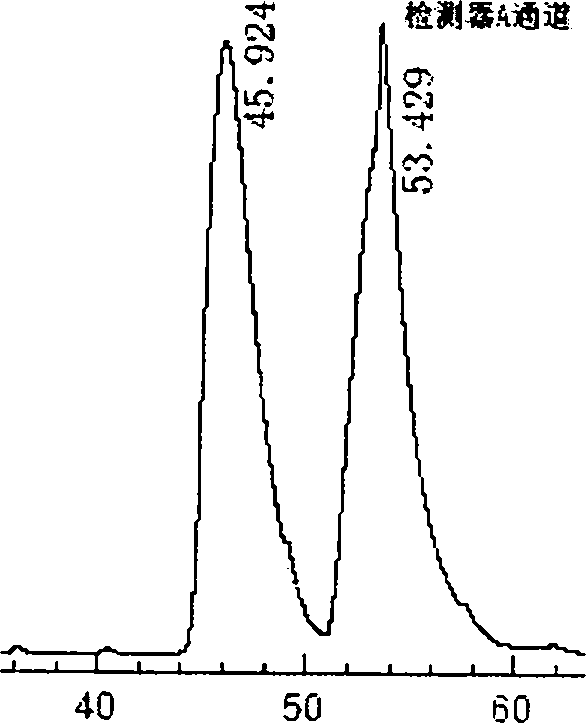
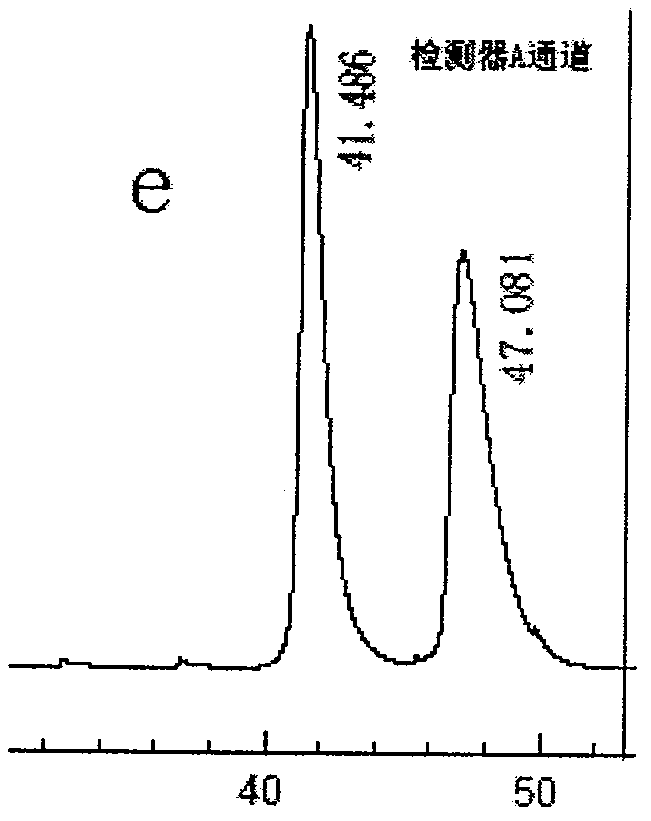
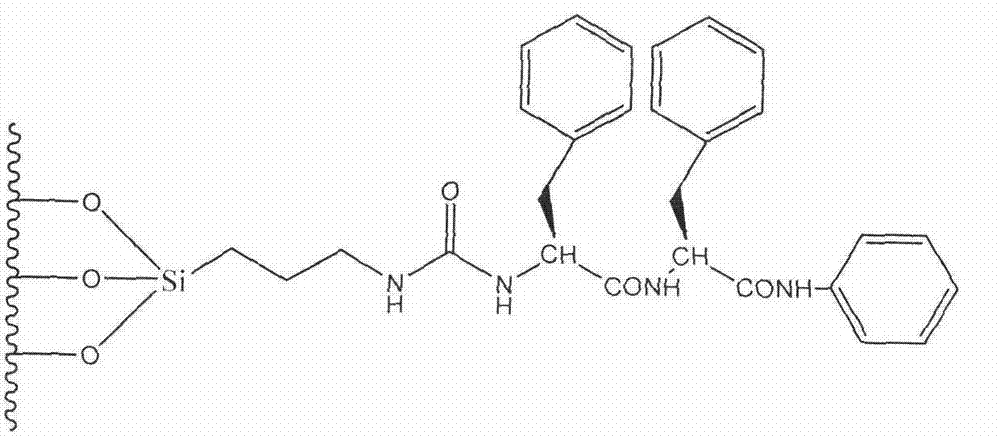
Preparation method of novel phenylalanine chiral chromatographic column stationary phase
A technology of chiral chromatographic column and phenylalanine, which is applied in the field of preparation of new chiral chromatographic column stationary phase, which can solve the problems of easy loss of stationary phase, tailing of effluent components, and limited service life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0040] Specific Example 1: Preparation of L-phenylalanine chiral stationary phase CSP1
[0041] 1. Pretreatment of the carrier:
[0042] Take 5.0g of silica gel balls, put them into an eggplant-shaped bottle, add 10mL of 12mol / L concentrated hydrochloric acid, heat to reflux for 2h, filter, wash with water until neutral, rinse with acetone and anhydrous ether in turn, and dry in vacuum. .
[0043] 2. Synthesis of Chiral Monomers
[0044] 1) Synthesis of Z-L-PheCOOH
[0045]Add 0.5 mol of NaOH to a three-neck flask containing 1.0 L of deionized water under ice-water bath, stir to dissolve, then add 0.5 mol of L-PheCOOH, stir to completely dissolve, add dropwise 0.5 mol of benzyloxychloride and 0.5 mol NaOH with the same volume of deionized aqueous solution, stirred at room temperature for 12 hours. Extract with ether, add concentrated hydrochloric acid to the extracted water phase, adjust the pH value to 2-3, add ethyl acetate for extraction, wash with water until neutral, ...
specific Embodiment 2
[0058] Specific Example 2: Preparation of L-phenylalanine chiral stationary phase CSP2
[0059] 1. Pretreatment of the carrier:
[0060] Take 5.0g of silica gel balls, put them into an eggplant-shaped bottle, add 10mL of 12mol / L concentrated hydrochloric acid, heat to reflux for 2h, filter, wash with water until neutral, rinse with acetone and anhydrous ether in turn, and dry in vacuum.
[0061] 2. Synthesis of Chiral Monomers
[0062] 1) Synthesis of Z-L-PheCOOH
[0063] Add 0.5 mol of NaOH to a three-neck flask containing 1.0 L of deionized water under ice-water bath, stir to dissolve, then add 0.5 mol of L-PheCOOH, stir to completely dissolve, add dropwise 0.5 mol of benzyloxychloride and 0.5 mol NaOH with the same volume of deionized aqueous solution, stirred at room temperature for 12 hours. Extract with ether, add concentrated hydrochloric acid to the extracted water phase, adjust the pH value to 2-3, add ethyl acetate for extraction, wash with water until neutral, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com