Polypyridine chiral ruthenium (II) complex and preparation method and application thereof
A technology of ruthenium complexes and polypyridines, applied in the field of polypyridine chiral ruthenium complexes and their preparation, can solve the problems of lack of systematic research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of the main ligand (dppz-idzo)
[0034] Take 0.27g (1.28mmol) of 1,10-phenanthroline-5,6-dione into a 125mL round bottom flask, add 50mL of ethanol, heat and stir to dissolve it completely, stop heating and cool to room temperature. After adding 0.21g (1.28mmol) of 5,6-diaminobenzimidazol-2-one to the above solution under magnetic stirring, a large amount of yellow precipitate was produced immediately, and the reaction was continued for 15 minutes under heating and reflux, and the product was filtered and collected. After drying, it could be The yellow powder dppz-idzo was obtained with a yield of 90.2%.
Embodiment 2
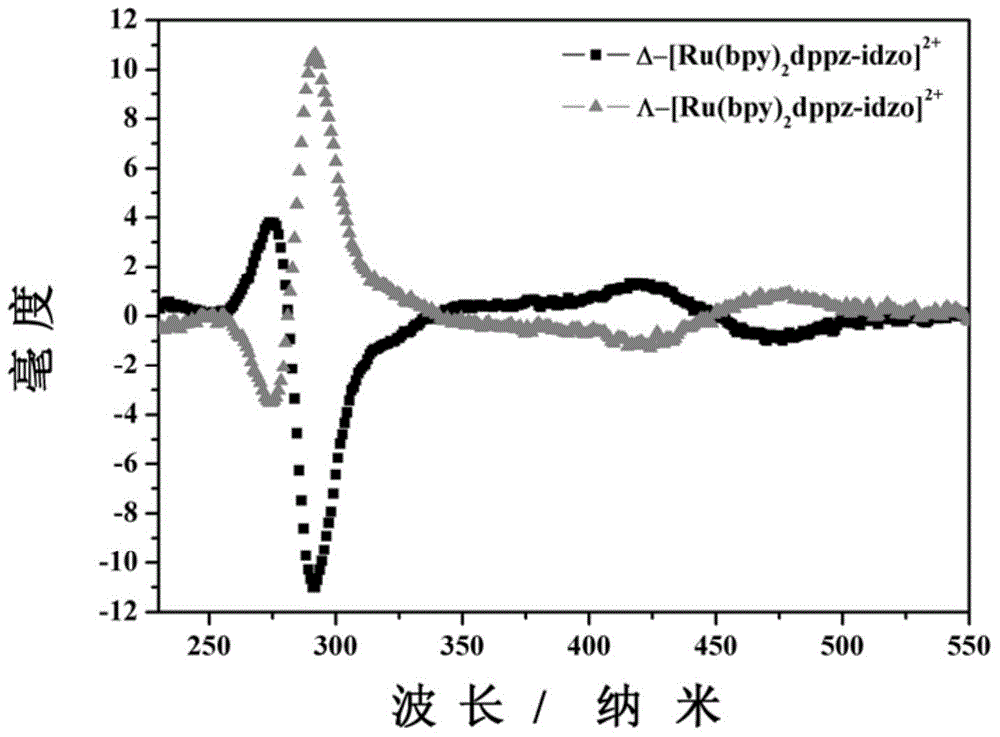
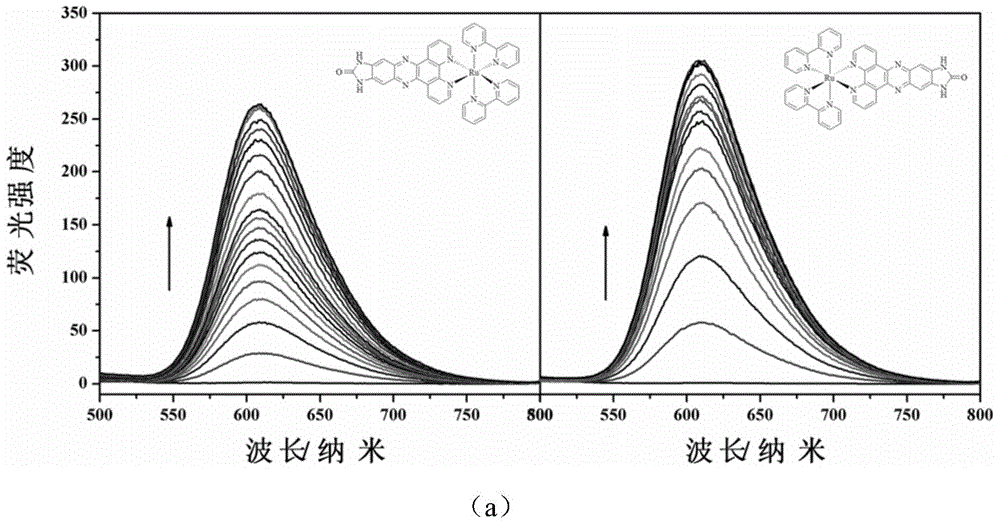
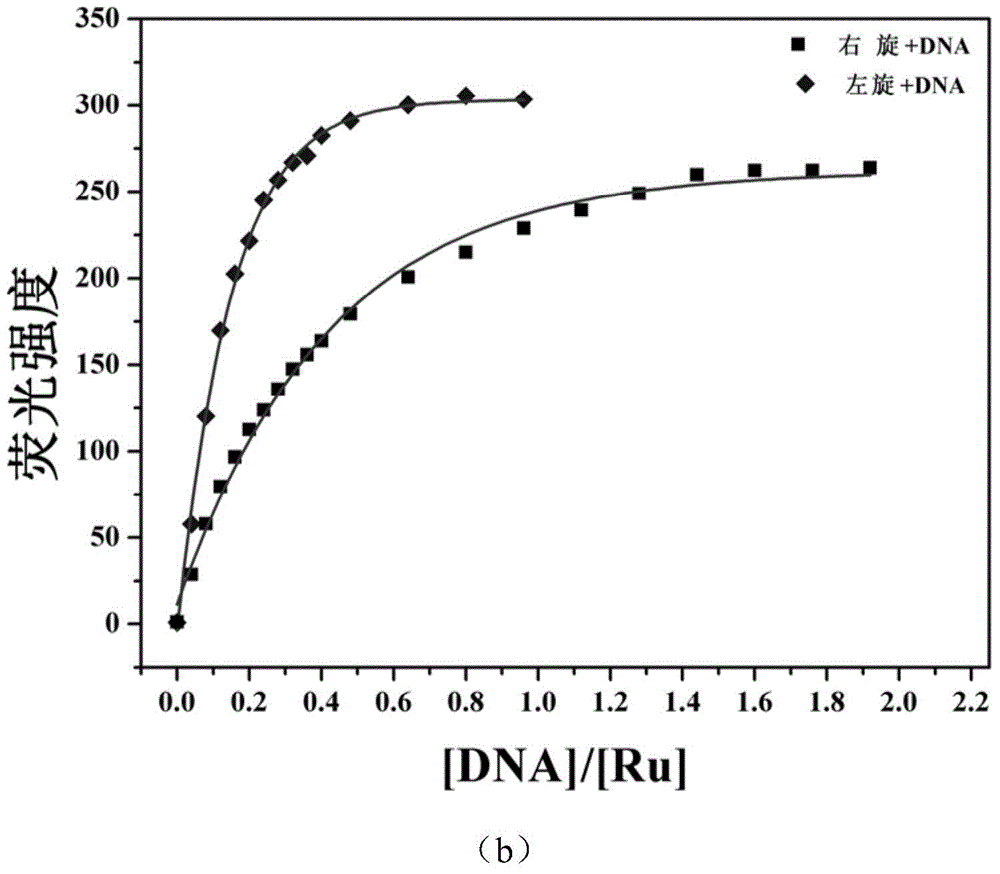
[0036] D-ruthenium (II) complexes (Δ-[Ru(bpy) 2 (dppz-idzo)](PF 6 ) 2 .2H 2 O) Synthesis
[0037] Add Δ-[Ru(bpy) to the pressure bottle 2 (py) 2 ].12H 2 O (130mg, 0.1mmol) and the main ligand (dppz-idzo) (21mg, 0.1mmol), using 20mL of a mixed solution of ethylene glycol and water (9:1, v / v) as a solvent, heated under the protection of argon Reflux 6h. After cooling to room temperature, add 40mL water to dilute, filter, and add saturated NH 4 PF 6 , filtered, washed, and dried to obtain a crude product. Then it was separated by neutral alumina chromatography column with acetonitrile and ethanol (5:1, v / v) as the eluent. Spin dry the eluent to get the product Δ-[Ru(bpy) 2 (dppz-idzo)](PF 6 ) 2 .2H 2 O.
[0038] Yield 73%, 1 H NMR [(CD 3 ) 2 SO]: δ11.73(s, 2H), 9.62(d, J=8.1Hz, 2H), 8.89(dd, J=13.0, 8.2Hz, 4H), 8.28-8.19(m, 4H), 8.14(t , J=7.6Hz, 2H), 8.01(dd, J=8.2, 5.4Hz, 2H), 7.84(d, J=5.3Hz, 2H), 7.76(d, J=5.3Hz, 4H), 7.61(t , J=6.4Hz, 2H), 7.38(t, J=6.4Hz...
Embodiment 3
[0042] L-ruthenium (II) complexes (Λ-[Ru(bpy) 2 (dppz-idzo)](PF 6 ) 2 .2H 2 O) Synthesis
[0043] The synthesis steps are similar to those of the D-ruthenium complex, and the Δ-[Ru(bpy) 2 (py) 2 ].12H 2 O is replaced by Λ-[Ru(bpy) 2 (py) 2 ].12H 2 O can get product product Λ-[Ru(bpy) 2 (dppz-idzo)](PF 6 ) 2 .2H 2 O.
[0044] Yield 75%, 1 H NMR [(CD 3 ) 2 SO]: δ11.71(s, 2H), 9.61(d, J=10.5Hz, 2H), 8.95-8.84(m, 4H), 8.17-8.28(m, 4H), 8.13(t, J=10.0Hz , 2H), 8.00(dd, J=10.5, 7.0Hz, 2H), 7.84(d, J=7.5Hz, 2H), 7.81-7.68(m, 4H), 7.60(t, J=8.0Hz, 2H) , 7.38(t, J=8.0Hz, 2H).Calc.for C 39 h 30 f 12 N 12 OP 2 Ru: C, 43.63; H, 2.82; N, 15.65. Found: C, 43.73; H, 2.83; N, 15.64. MALDI-MS: calcd.For C 39 h 26 N 10 ORu 751.76[M] + ;found 751.1[M] + .
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com