Nitrile hydratase and application thereof
A technology for nitrile hydratase and nitrile compounds, which is applied in the field of genetic engineering and can solve the problems of accumulation of carboxylic acid compounds, intermittency, and unstable product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
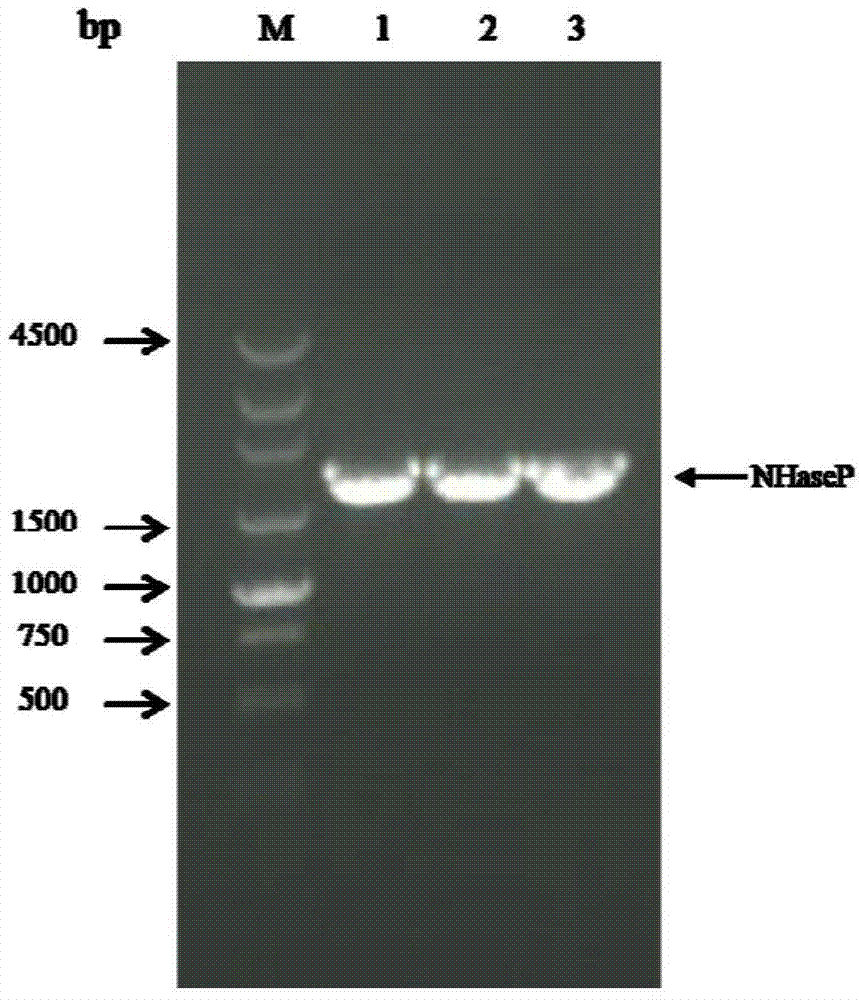
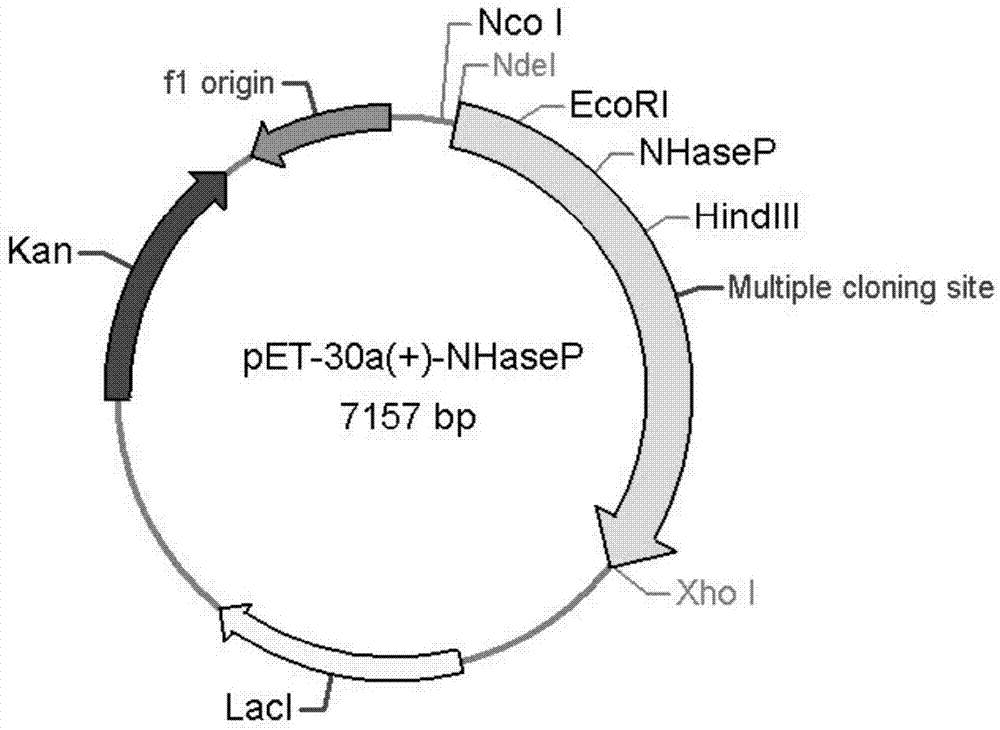
[0049] 1. Cloning of nitrile hydratase gene and activator gene from Bordetella petrii DSM 12804 genome
[0050] Primers NP-F (upstream primer) and NP-R (downstream primer) were designed according to the Bordetella DSM 12804 genomic DNA sequence (GenBank accession number: AM902716.1).
[0051] NP-F sequence: 5'-CCG GAATTC ATGCTCGAAGTTCTTTGCATGG-3'
[0052] NP-R sequence: 5'-TTCCC AAGCTT GTTAAGCCATTGCGGCAACG-3'
[0053] Restriction sites EcoRI and HindIII (underlined) were added to the upstream and downstream primers, respectively. Using Bordetella DSM 12804 genomic DNA as template, NP-F and NP-R as primers for PCR amplification, the PCR reaction system and reaction conditions are as follows:
[0054] PCR amplification system:
[0055]
[0056]
[0057] PCR amplification conditions:
[0058] 1) Pre-denaturation: 95°C for 5 minutes;
[0059] 2) Denaturation: 98°C for 10s; Annealing: 56°C for 15s; Extension: 72°C for 100s; a total of 30 cycles;
[0060] 3) Extensio...
Embodiment 2
[0071] Example 2 Genetically engineered bacteria catalyze acrylonitrile to generate acrylamide
[0072] The enzyme activity unit is defined as: under the reaction conditions, the amount of enzyme that catalyzes the substrate reaction to produce 1 μmol of product per minute.
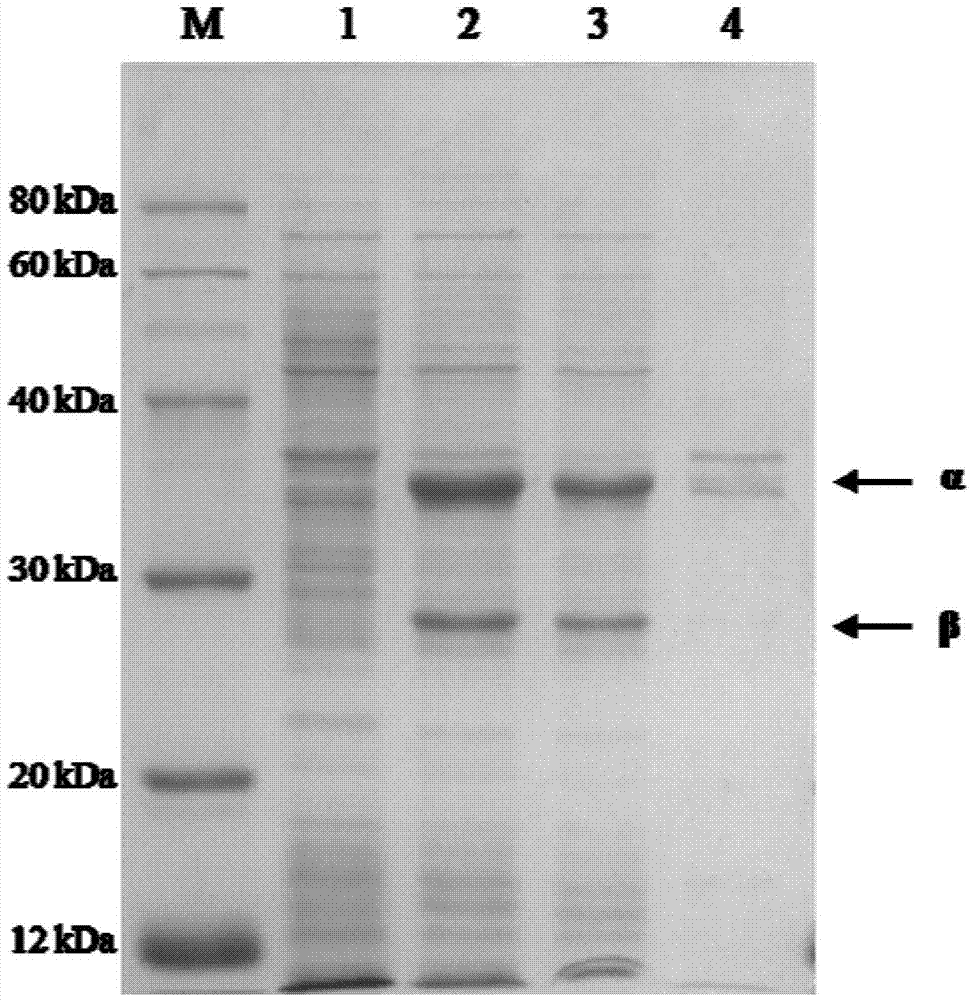
[0073] Get the fermented liquid of the engineered bacterium E.coli BL21(DE3) / pET-30a(+)-NHaseP that 25ml embodiment 1 constructs, 10000rpm, 10min centrifugal collection thalline, then use the buffering of 250ml 50mM Tris-HCl (pH 8.5) The collected bacterial cells were resuspended in the liquid, and the enzyme activity of the resuspended enzyme solution was 1700U / ml. Add 1.0ml of acrylonitrile to the resuspension, and carry out hydration reaction at 20°C for 120min. Then the contents of acrylonitrile, acrylamide and acrylic acid in the reaction system were detected by gas chromatography. The conversion rate of the substrate is greater than 99%, the yield is greater than 92%, and no acrylic acid is found ...
Embodiment 3
[0074] Example 3 Genetically engineered bacteria catalyze 3-cyanopyridine to generate nicotinamide
[0075] Get the fermented liquid of the engineered bacterium E.coli BL21(DE3) / pET-30a(+)-NHaseP that 25ml embodiment 1 constructs, 10000rpm, 10min centrifugal collection thalline, then use the buffering of 250ml 50mM Tris-HCl (pH 6.0) The collected bacteria were resuspended. Add 5.0 g of 3-cyanopyridine to the resuspension, and carry out hydration reaction at 37° C. for 120 min. Then use high performance liquid chromatography to detect the content of 3-cyanopyridine and nicotinamide in the reaction system. The substrate conversion rate is greater than 99%, and the yield is greater than 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com