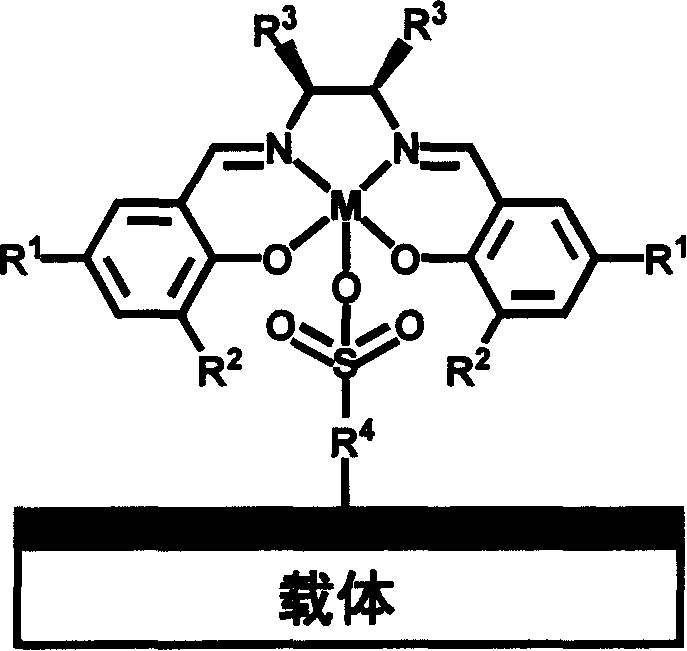
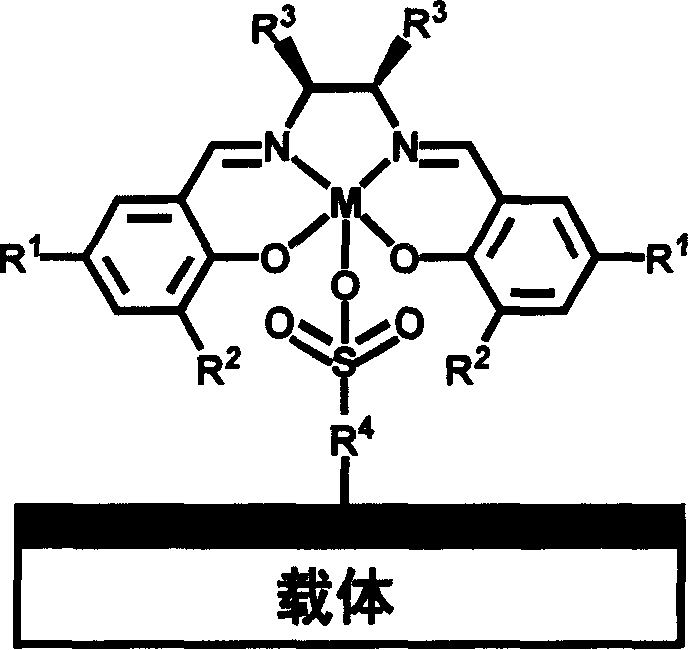
Chiral schiff base-metal heterogeneous epoxidation catalyst and its prepn. method
A technology of oxidation catalysts and Schiff bases, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., can solve complex catalyst preparation, loss, activity and ee value Poor problems, easy to purify, easy to prepare, easy to handle and operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1) 3 grams of MCM-41 (pore size 1.6nm) was heated and vacuum-treated by adding 150ml of anhydrous toluene and 6ml of HS (CH 2 ) 3 Si(OMe) 3 , Grafted at 120°C for 18h, then filtered, washed and dried.
[0046] (2) Then use 30% H 2 o 2 27ml of the precursor obtained in oxidation step 1 for 24h, add 0.33g NaHCO after filtering and washing 3 20ml of aqueous solution was reacted at room temperature for 3h, filtered, washed and dried.
[0047] (3) the complex (1.0mmol, wherein R 1 =t-Bu,R 2 =t-Bu,R 3 =Ph, chiral ortho-diamine in (1S,2S) configuration, metal =Mn) for 5 hours. The number of heterogeneous catalysts that can be prepared is A.
Embodiment 2
[0049] Same as Example 1, except that step 1 is changed: the carrier adopts an organic-inorganic hybrid material containing propane mercapto group [synthesis details see Micro.Meso.Mater.77 (2005) 257], and step 3 uses Complexes of M(salen) (where R 1 =t-Bu, R 2 =t-Bu, R 3 =-(CH 2 ) 4 -, the chiral adjacent diamine is (1R, 2R) configuration, M=Mn), and the heterogeneous catalyst number of making is B.
Embodiment 3
[0051] (1) Add 150ml of anhydrous toluene and 7ml of PhSi(OEt) to 3 grams of SBA-15 (pore size 7.6nm) after heating and vacuum treatment 3 , Grafted at 120°C for 18h, then filtered, washed and dried. (2) Heat and vacuum the precursor obtained in step 1, add 10 ml of concentrated sulfuric acid for sulfonation at 100 degrees for 8 hours, then filter, wash and dry. Then with 0.33g NaHCO 3 20ml of the aqueous solution was reacted at room temperature for 3 hours, then filtered, washed and dried. (3) the catalyst precursor obtained in step 2 is refluxed in the ethanol of 60ml to graft the complex of M (salen) (1.0mmol, wherein R 1 =t-Bu, R 2 =t-Bu, R 3 =-(CH 2 ) 4 -, chiral ortho-diamine is (1S, 2S) configuration, M=Mn) the heterogeneous catalyst that can be prepared in 5 hours, numbering is C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com