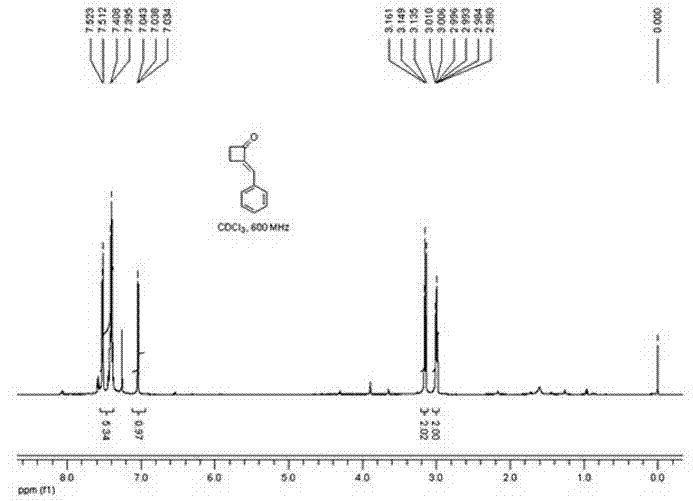
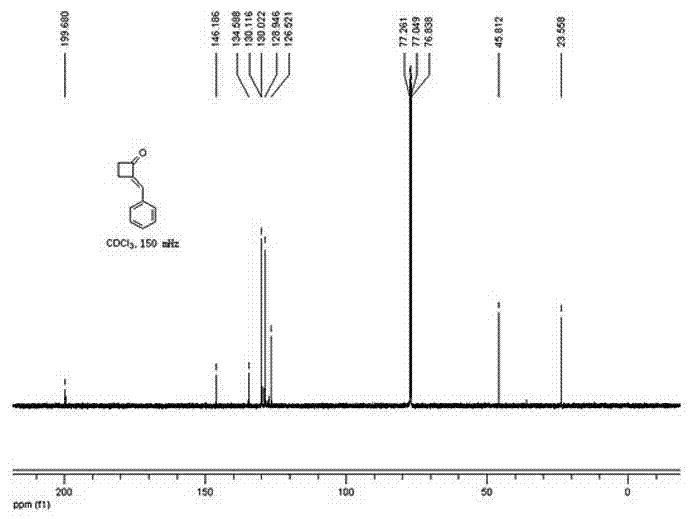
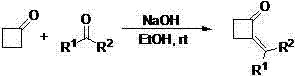Method for preparing 2-alkylene cyclobutanone
A technology of hydrocarbylene cyclobutanone and hydrocarbyl cyclobutanone, which is applied in the field of synthesis of new synthetic building block methylene cyclobutanone, can solve the problems of long route, limited application, harsh conditions required for reaction, etc., and achieve high efficiency , short route and low catalyst cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 1. Reaction formula:
[0020]
[0021] 2. The amount and properties of the reaction raw materials:
[0022] substance molecular weight millimoles Mass / g Volume / mL Cyclobutanone 70 30 2.1 Benzaldehyde 106 10 1.06 sodium hydroxide 40 0.2 0.008 ethanol 10
[0023] 3. Operation process:
[0024] In a 50 mL round bottom flask, add cyclobutanone, benzaldehyde, ethanol, and sodium hydroxide in sequence, and stir at room temperature at 20°C (the dosage is shown in the table above). The reaction solution gradually turned light yellow. The reaction was monitored by thin layer chromatography (developing solvent: petroleum ether: ethyl acetate 5:1).
[0025] After the reaction was completed for 1 h, the reaction liquid was poured into a mixture of 25 mL ethyl acetate and 30 mL water for extraction, and the aqueous layer was extracted twice with ethyl acetate (20 mL ethyl acetate each time). The organic la...
Embodiment 2
[0028] 1. Reaction formula:
[0029]
[0030] two,
[0031] Numbering R 1 R 2 Yield(%) 1 p -MeC 6 h 4 H 74 2 p -MeOC 6 h 4 H 80 3 p -ClC 6 h 4 H 56 4 C 7 h 15 H 33 5 C 6 h 5 CH 3 53 6 C 6 h 5 C 6 h 5 58 7 C 3 h 7 CH 3 23
[0032] Replace benzaldehyde with other aldehydes and ketones, and other conditions are the same as in Example 1, and the experimental results are as shown in the table above.
[0033] The above table shows that this method has a wide range of applications, and both aldehydes and ketones can be used to synthesize corresponding compounds.
Embodiment 3
[0035] solvent water Methanol Isopropanol Product yield (%) 47 60 64
[0036] Replace ethanol with other solvents, and other conditions are the same as Example 1, and the experimental results are shown in the table above.
[0037] The above table shows that under the same other conditions, the yield of ethanol is the highest.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com