A method for preparing pinoxaden by "one pot method"
A technology of pinoxaden and pot cooking, which is applied in the field of "one pot cooking" to prepare pinoxaden, can solve the problems of increasing product production cost and environmental protection pressure, increasing the complexity of production operations, and long online reaction time, etc., to achieve reduction The effect of online reaction time, reduction of three wastes, and increase of conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
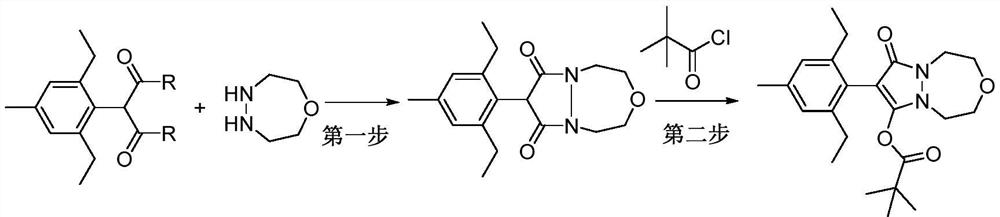
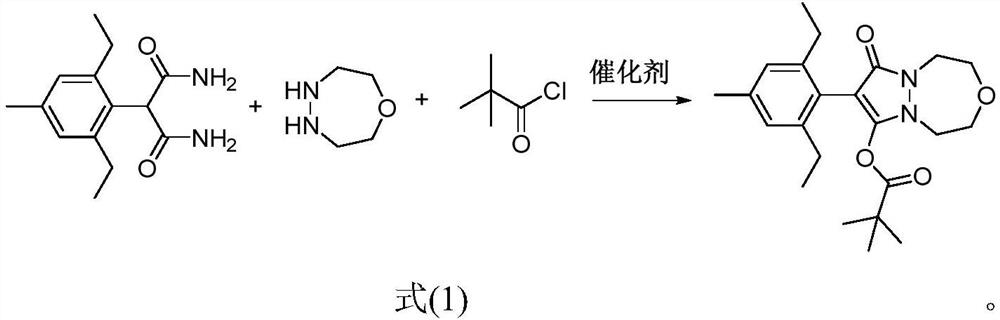
[0021] 2-(2,6-diethyl-4-methylphenyl) malonamide (2.5g, 10mmol), 1-oxo-4,5-diazepane (1.0g, 10mmol) and Catalytic amount (0.5mmol) of nano-magnesium oxide was added to 100mL of toluene, stirred and heated to 120°C, reacted for 10h, then cooled the reaction solution to room temperature, added triethylamine (2.0g, 20mmol), stirred, and then dripped while stirring Add pivaloyl chloride (1.4g, 12mmol), after dropping, continue to stir in the greenhouse for 5h, filter with suction, wash the filtrate with 5% hydrochloric acid aqueous solution and 10% sodium hydroxide aqueous solution respectively, separate the organic phase, dry, and filter The desiccant was removed, concentrated, and recrystallized from n-hexane to obtain 2.8 g of pinoxaden, with a yield of 70%.
[0022] MS(ESI)m / z[M+H] + = 401.2.
[0023] 1 H NMR (CDCl 3 )δ: 1.03(s,9H), 1.12(t,6H), 2.29(s,3H), 2.35~2.63(m,4H), 3.81~3.90(m,4H), 3.93(m,2H), 4.26 (m,2H), 6.88(s,2H).
Embodiment 2
[0025] 2-(2,6-diethyl-4-methylphenyl) malonamide (2.5g, 10mmol), 1-oxo-4,5-diazepane (1.0g, 10mmol) and Add a catalytic amount (0.5mmol) of nano-titanium oxide to 100ml of toluene, stir and heat to 120°C, react for 10h, then cool the reaction solution to room temperature, add triethylamine (2.0g, 20mmol), stir, and then drop while stirring Add pivaloyl chloride (1.4g, 12mmol), after dropping, continue to stir in the greenhouse for 5h, filter with suction, wash the filtrate with 5% hydrochloric acid aqueous solution and 10% sodium hydroxide aqueous solution respectively, separate the organic phase, dry, and filter The desiccant was removed, concentrated, and recrystallized from n-hexane to obtain 2.9 g of pinoxaden, with a yield of 72%.
[0026] MS(ESI)m / z[M+H] + = 401.2.
[0027] 1 H NMR (CDCl 3 )δ: 1.03(s,9H), 1.12(t,6H), 2.29(s,3H), 2.35~2.63(m,4H), 3.81~3.90(m,4H), 3.93(m,2H), 4.26 (m,2H), 6.88(s,2H).
Embodiment 3
[0029] 2-(2,6-diethyl-4-methylphenyl) malonamide (2.5g, 10mmol), 1-oxo-4,5-diazepane (1.0g, 10mmol) and Catalytic amount (0.5mmol) of nano-zirconia was added to 100ml of dioxane, stirred and heated to 110°C, reacted for 10h, then cooled the reaction solution to room temperature, added triethylamine (2.0g, 20mmol), stirred, and then Add pivaloyl chloride (1.8 g, 15 mmol) dropwise while stirring, continue to stir in the greenhouse for 5 h, filter with suction, wash the filtrate with 5% aqueous hydrochloric acid solution and 10% aqueous sodium hydroxide solution, and separate the organic phase. The desiccant was removed by filtration, filtered, concentrated, and recrystallized from n-hexane to obtain 2.4 g of pinoxaden with a yield of 60%.
[0030] MS(ESI)m / z[M+H] + = 401.2.
[0031] 1 H NMR (CDCl 3 )δ: 1.03(s,9H), 1.12(t,6H), 2.29(s,3H), 2.35~2.63(m,4H), 3.81~3.90(m,4H), 3.93(m,2H), 4.26 (m,2H), 6.88(s,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com