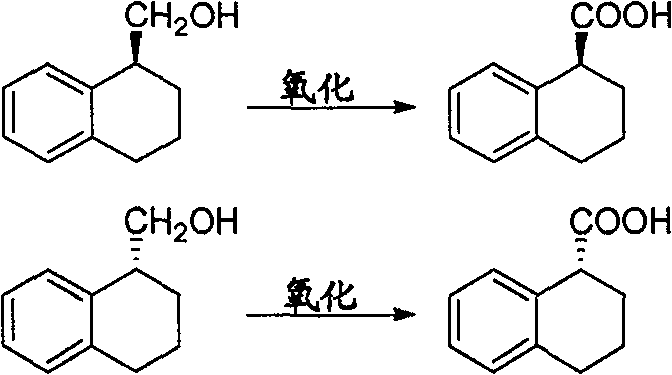
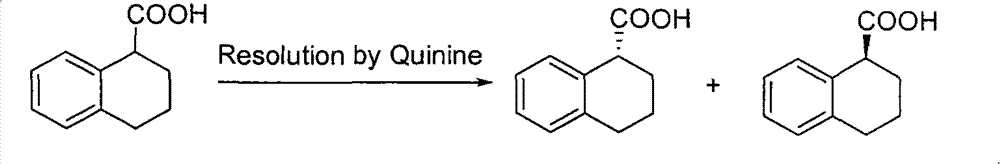
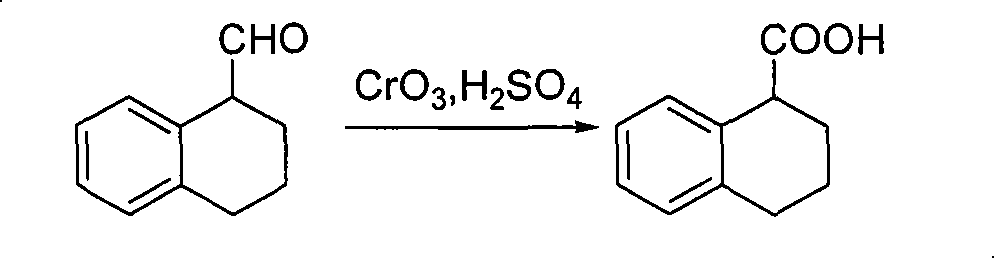
Method for preparing chiral 1, 2, 3, 4-tetrahydro-1-naphthoic acid
A naphthoic acid and chiral technology, which is applied in the field of preparation of chiral 1, can solve the problems of serious environmental pollution of chromium trioxide oxidant, and achieve the effects of easy separation and purification, mild conditions and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of (S)-1,2,3,4-tetrahydro-1-naphthoic acid
[0027] Put 5.00g (S)-1,2,3,4-tetrahydro-1-naphthalenemethanol into 100ml acetone, cool to -78°C in an ice-water bath, add 20ml Jones reagent dropwise under stirring (dissolved by 5.34g chromium trioxide in 4.6ml of concentrated sulfuric acid and diluted to 20ml with water to obtain), about 1 hour to complete the dropwise reaction, heat preservation reaction for 2 hours, the reaction solution was poured into about 30ml of ice water, after fully stirring, extracted with dichloromethane (20ml×3), combined The organic layer was washed with water (20ml×3), then the organic layer was extracted with 5% aqueous sodium hydroxide solution (20ml), the sodium hydroxide layer was washed with ethyl acetate (20ml), and the pH was adjusted to 2-3 by adding concentrated HCl, and then Extracted with dichloromethane (15ml×3), combined the organic layers, dried with anhydrous magnesium sulfate, and recovered the solvent under reduced...
Embodiment 2
[0031] Preparation of (R)-1,2,3,4-tetrahydro-1-naphthoic acid
[0032] 5.00g (S)-1,2,3,4-tetrahydro-1-naphthalenemethanol was dropped into 50ml of dichloromethane, cooled in an ice-water bath to -78°C, and 20ml of Jones reagent (made from 5.34g of chromium trioxide) was added dropwise with stirring. Dissolve in 4.6ml of concentrated sulfuric acid and dilute to 20ml with water to obtain), add dropwise in about 1 hour, keep warm for 2 hours, pour the reaction solution into about 30ml of ice water, stir well and separate the organic layer, and the water layer with dichloromethane (15ml×2) extraction, combined the organic layers and washed with water (20ml×3), then extracted the organic layer with 5% aqueous sodium hydroxide solution (20ml), washed the sodium hydroxide layer with ethyl acetate (20ml) and added concentrated HCl Adjust the pH to 2-3, then extract with dichloromethane (15ml×3), combine the organic layers, dry over anhydrous magnesium sulfate, and recover the solvent ...
Embodiment 3
[0036] Preparation of (S)-1,2,3,4-tetrahydro-1-naphthoic acid
[0037] Put 4.00g (S)-1,2,3,4-tetrahydro-1-naphthalenemethanol into 200ml acetone, 60ml carbonate buffer solution, cool to -20°C in an ice-water bath, add 0.49g sodium bromide, 0.08 g2,2,6,6-tetramethyl nitroxide radical piperidine (TEMPO), add 3.50 g of trichloroisocyanuric acid (TCCA) under stirring, finish adding in about 30 minutes, keep warm for 1.5 hours, add 12ml of isocyanuric acid Propanol, filter, evaporate about 1 / 2 of the solvent under reduced pressure, add 60ml of saturated aqueous sodium carbonate solution, then extract with ethyl acetate (50ml), separate the water layer and add concentrated hydrochloric acid to adjust the pH value to 2~3, and use ethyl acetate (50ml×2) extraction, the combined organic phases were extracted with 5% NaOH aqueous solution, the aqueous layer was added concentrated hydrochloric acid to adjust the pH value to 2-3, extracted with dichloromethane (15ml×2), the combined organ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com