A treating method of a ganciclovir condensation compound isomer
A technology of ganciclovir condensate and processing method, which is applied in the field of chemical substance recovery to achieve the effects of reducing environmental pollution, reducing raw material cost and improving total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
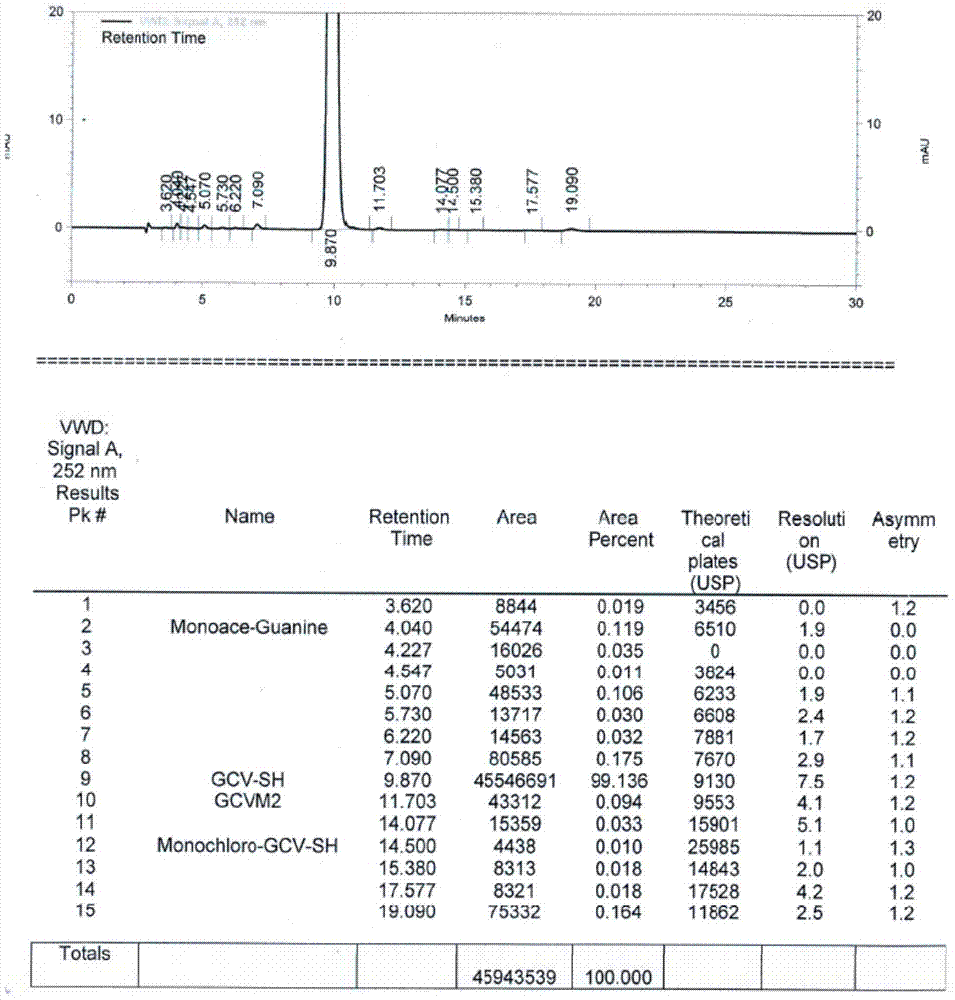
[0031] Put 40g of ganciclovir condensate isomers, 2g of p-toluenesulfonic acid and 160ml of DMF into the reaction flask, add 5ml of acetic anhydride, and keep the reaction at 120-130°C for 45h. After the reaction, the solvent was concentrated under reduced pressure, and 320ml of methanol was added to dissolve the residue. After the solution was clear, it was cooled to 20-25°C, and stirred for 3h to crystallize. After filtering, the filtrate was concentrated under reduced pressure, and the concentrated residue was dissolved in a mixed solvent composed of 30ml of methanol and 170ml of toluene, and then cooled to 5-10°C for crystallization for 2h. Filter, add the filter cake to 200ml of methanol, stir at 50-60°C for 1 hour, cool to 5-15°C and stir for 1 hour. Filter and dry to obtain 20.8g of ganciclovir condensate, as attached figure 1 As shown, GCVM2 is an isomer of ganciclovir condensate, the purity of ganciclovir condensate is 99.136%, and the isomer content is 0.094%.
Embodiment 2
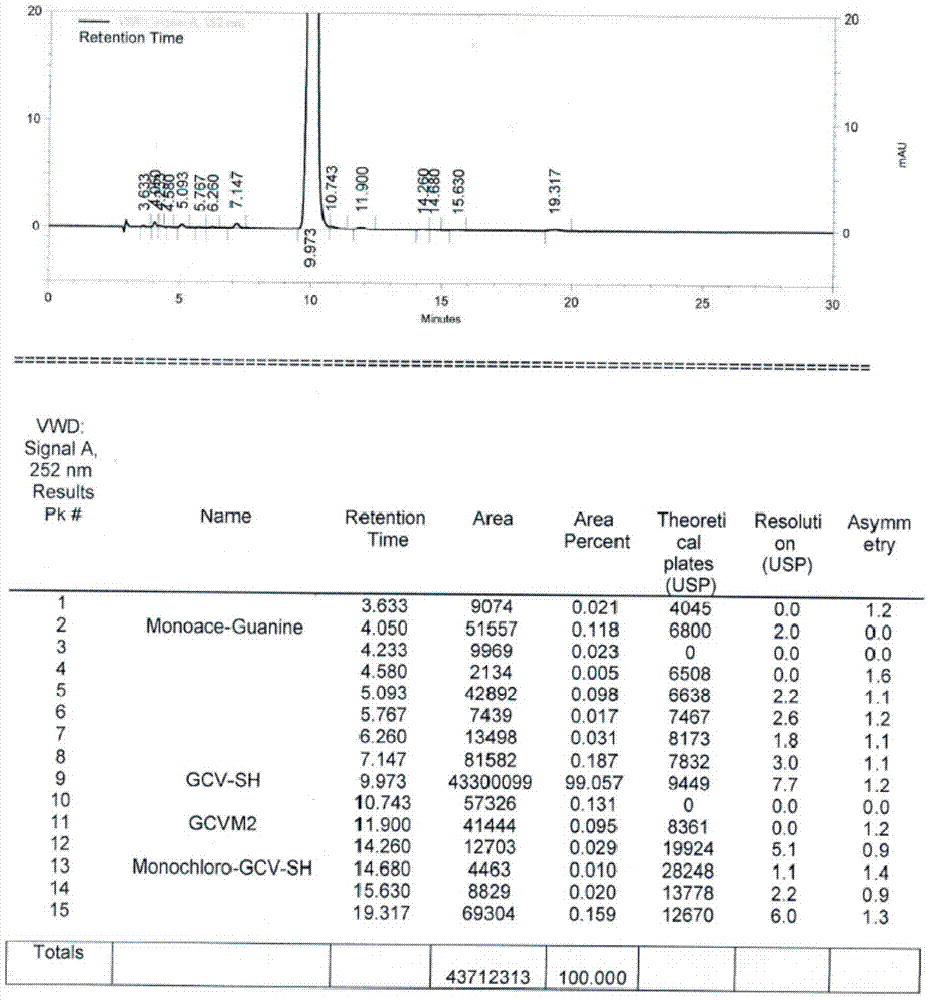
[0033] Put 40g of ganciclovir condensate isomers, 2g of p-toluenesulfonic acid and 160ml of DMSO into the reaction flask, add 5ml of acetic anhydride, and keep the reaction at 120-130°C for 45h. After the reaction, the solvent was concentrated under reduced pressure, and 320ml of methanol was added to dissolve the residue. After the solution was clear, it was cooled to 20-25°C, and stirred for 3h to crystallize. After filtering, the filtrate was concentrated under reduced pressure, and the concentrated residue was dissolved in a mixed solvent composed of 30ml of methanol and 170ml of toluene, and then cooled to 5-10°C for crystallization for 2h. Filter, add the filter cake to 200ml of methanol, stir at 50-60°C for 1 hour, cool to 5-15°C and stir for 1 hour. Filter and dry to obtain 19.8g of ganciclovir condensate, as attached figure 2 As shown, GCVM2 is an isomer of ganciclovir condensate, the purity of ganciclovir condensate is 99.057%, and the isomer content is 0.095%.
Embodiment 3
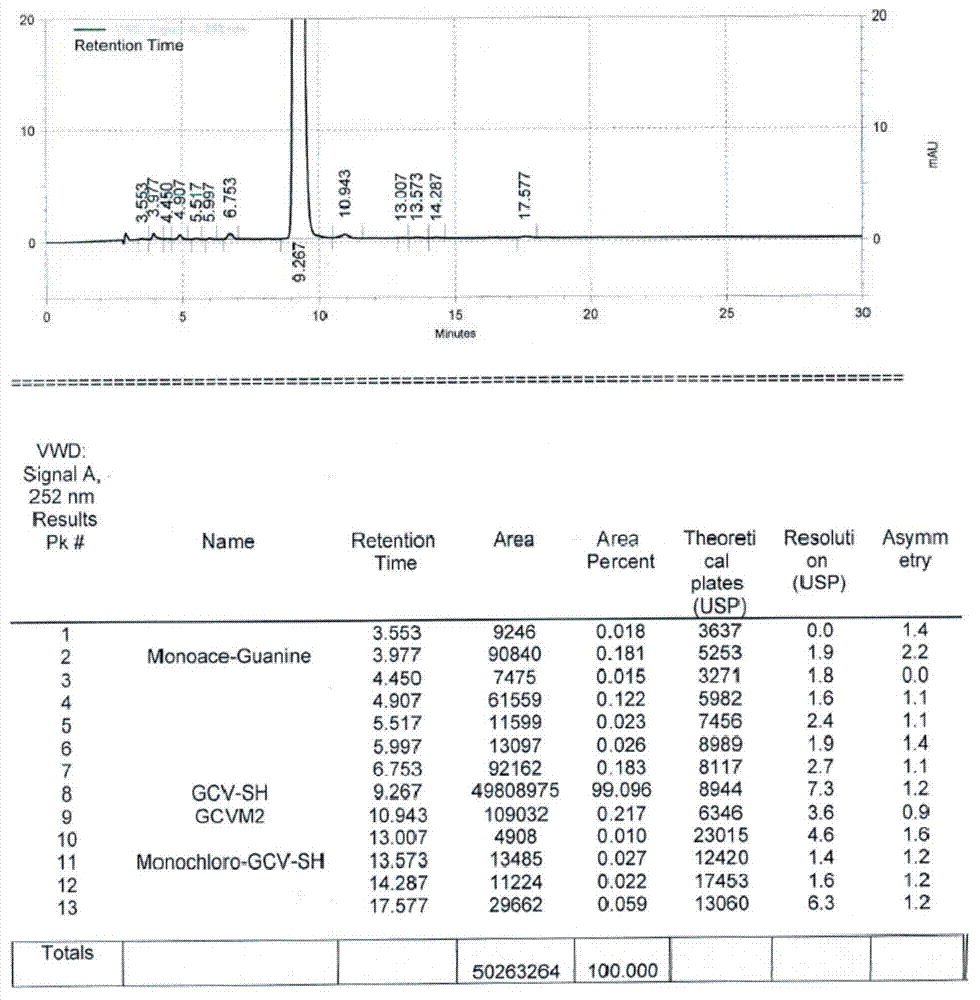
[0035] Put 40g of ganciclovir condensate isomers, 2g of p-toluenesulfonic acid and 160ml of formamide into the reaction flask, add 5ml of acetic anhydride, and keep the reaction at 120-130°C for 45h. After the reaction was completed, the solvent was concentrated under reduced pressure, and 320ml of methanol was added to dissolve the residue. After dissolving, cool to 20-25°C and stir for 3h to crystallize. After filtering, the filtrate was concentrated under reduced pressure, and the concentrated residue was dissolved in a mixed solvent composed of 30ml of methanol and 170ml of toluene, and then cooled to 5-10°C for crystallization for 2h. Filter, add the filter cake to 200ml of methanol, stir at 50-60°C for 1 hour, cool to 5-15°C and stir for 1 hour. Filter and dry to get ganciclovir condensate 20.2g, as attached image 3 As shown, GCVM2 is an isomer of ganciclovir condensate, the purity of ganciclovir condensate is 99.096%, and the isomer content is 0.217%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com