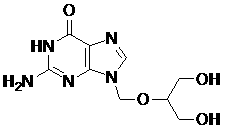
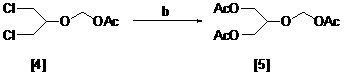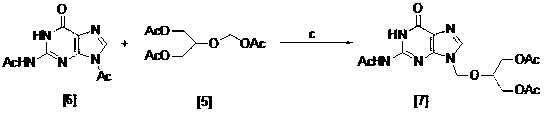Method for preparing ganciclovir
A technology of ganciclovir and acetoxymethoxypropane, applied in the field of preparation of ganciclovir, can solve problems such as needs, and achieve the effects of reducing reaction steps, high product yield and cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of 1,3-Dichloro-2-acetoxymethoxypropane [4]
[0034] Add 100g of 1,3-dichloroglycerin, 24g of paraformaldehyde and 1g of methanesulfonic acid in turn, stir and heat to 85 oC . After 6 hours of reaction, cool to 10 oC , 158g of acetic anhydride was started to be dripped, and after the dripping, the stirring was continued for 6 hours, and the mixture was allowed to stand overnight. Liquid separation, drying, and distillation to obtain the product 1,3-dichloro-2-acetoxymethoxypropane [4] 65g. The weight yield is 65%, and the content is 97%.
[0035] Preparation of 1,3-Diacetoxy-2-acetoxymethoxypropane [5]
[0036] Add 10g of 1,3-dichloro-2-acetoxymethoxypropane, 25ml of N,N-dimethylacetamide, and 4ml of acetic anhydride to the reaction flask, and add 25g of anhydrous potassium acetate under stirring. , Warming to 80 oC Add 0.2g of tetrabutylammonium bromide, adjust the temperature to 140°C, start timing, and keep the reaction for 24 hours. Cool down, filter by s...
Embodiment 2
[0043] Preparation of 1,3-Dichloro-2-acetoxymethoxypropane [4]
[0044] Add 100g of 1,3-dichloroglycerin, 28g of paraformaldehyde and 1g of p-toluenesulfonic acid in sequence, stir and heat to 105 oC . After 2 hours of reaction, cool to 40 oC , Start dripping 80g of acetic anhydride, continue to stir for 4 hours after dripping, and let stand overnight. Liquid separation, drying, and distillation to obtain product 1,3-dichloro-2-acetoxymethoxypropane [4] 68g. The weight yield is 68%, and the content is 97%.
[0045] Preparation of 1,3-Diacetoxy-2-acetoxymethoxypropane [5]
[0046] Add 10g of 1,3-dichloro-2-acetoxymethoxypropane, 30ml of N,N-dimethylformamide, and 8ml of acetic anhydride to the reaction flask, and add anhydrous sodium acetate 11.3 under stirring. g, increase the temperature, add 0.4g of tetramethylammonium bromide, then continue to adjust the temperature to 100°C, start timing, and keep the reaction for 30 hours. Cool down, filter by suction, and wash the filter c...
Embodiment 3
[0058] Preparation of 1,3-Dichloro-2-acetoxymethoxypropane [4]
[0059] Add 100g of 1,3-dichloroglycerin, 100g of paraformaldehyde and 3g of concentrated sulfuric acid in turn, stir and heat to 110 oC . After 4 hours of reaction, cool to 20 oC Begin to add 380g of acetic anhydride, and continue to stir for 8 hours after the addition, and let it stand overnight. Liquid separation, drying, and distillation to obtain the product 1,3-dichloro-2-acetoxymethoxypropane [4] 70g. The weight yield is 70%, and the content is 98%.
[0060] Preparation of 1,3-Diacetoxy-2-acetoxymethoxypropane [5]
[0061] Add 10g of 1,3-dichloro-2-acetoxymethoxypropane, dimethyl sulfoxide solvent, and 2.35ml of acetic anhydride into the reaction flask accordingly, add 8g of anhydrous sodium acetate under stirring, increase the temperature, and add Tetramethylammonium bromide 7g, then adjust the temperature to 120°C, start timing, and keep the reaction for 18 hours. Cool down, filter by suction, and wash the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com