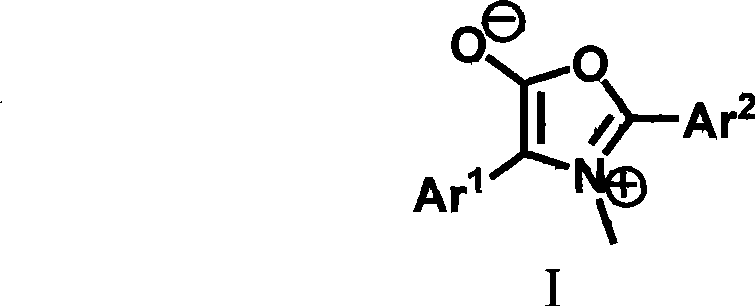
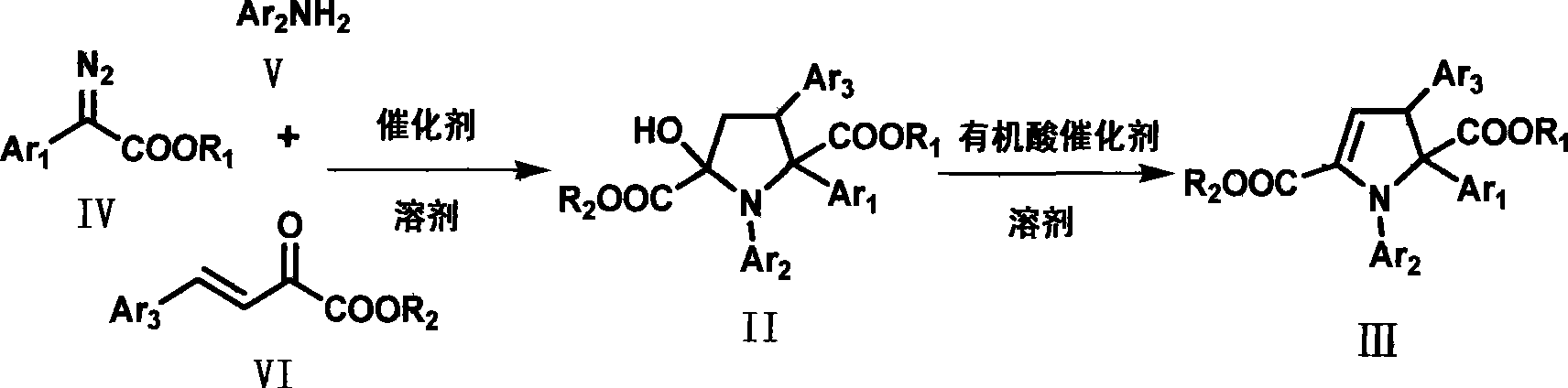
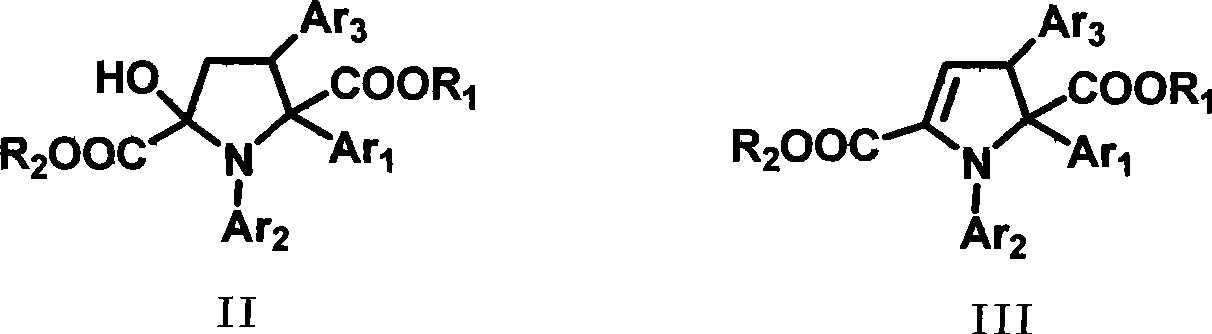Dihydropyrrole derivates and method for preparing intermediate pyrrolidine
A technology of tetrahydropyrrole and dihydropyrrole, which is applied in the direction of organic chemistry, can solve the problems of unsuitability for industrialization, inconvenient operation, site selectivity and low yield, and achieve high reaction yield and safe and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Dissolve (E)-2-oxo-4-p-chlorophenyl-3-butenoic acid methyl ester (2mmol), aniline (2.4mmol) and rhodium acetate (0.02mmol) in dichloromethane (12mL) to form For the reaction system, dissolve methyl phenyldiazoacetate (2.4mmol) in 5mL of dichloromethane to form a solution, and slowly add the solution formed by dissolving methyl phenyldiazoacetate in dichloromethane under reflux within 1 hour into the reaction system. After the dropwise addition, the solvent was evaporated under reduced pressure to obtain a crude product, which was subjected to column chromatography (ethyl acetate:petroleum ether=1:40~1:10) to obtain the corresponding intermediate tetrahydropyrrole derivative, and the yield was was 78%.
[0030] The intermediate tetrahydropyrrole derivative (1mmol) was mixed with toluene (15mL), citric acid monohydrate (0.2mmol) was added, water was carried through the water separator under reflux for 3 hours, cooled to room temperature, and the solvent was evaporated un...
Embodiment 2
[0032] Dissolve (E)-methyl 2-oxo-4-m-bromophenyl-3-butenoate (2mmol), aniline (2.4mmol) and rhodium acetate (0.02mmol) in dichloromethane (12mL) to form For the reaction system, methyl phenyldiazoacetate (2.4 mmol) was dissolved in 5 mL of dichloromethane to form a solution, and the solution was slowly added dropwise to the reaction system within 1 hour under reflux. After the dropwise addition, the solvent was evaporated under reduced pressure to obtain a crude product, which was subjected to column chromatography (ethyl acetate:petroleum ether=1:40~1:10) to obtain the corresponding intermediate tetrahydropyrrole derivative, and the yield was was 73%.
[0033] The intermediate tetrahydropyrrole derivative (1mmol) was mixed with toluene (15mL), and citric acid monohydrate (0.2mmol) was added, and the reaction system was refluxed with water for 4 hours through a water separator, cooled to room temperature, and depressurized The solvent was evaporated to obtain a crude product,...
Embodiment 3
[0035]Dissolve (E)-methyl 2-oxo-4-p-nitrophenyl-3-butenoate (2mmol), aniline (2.4mmol) and rhodium acetate (0.02mmol) in dichloromethane (12mL) To form a reaction system, methyl phenyldiazoacetate (2.4 mmol) was dissolved in 5 mL of dichloromethane to form a solution, and the solution was slowly added dropwise to the reaction system within 1 hour under reflux. After the dropwise addition, the solvent was evaporated under reduced pressure to obtain a crude product, which was subjected to column chromatography (ethyl acetate:petroleum ether=1:40~1:10) to obtain the corresponding intermediate tetrahydropyrrole derivative, and the yield was was 86%.
[0036] The intermediate tetrahydropyrrole derivative (1mmol) was mixed with toluene (15mL), and citric acid monohydrate (0.2mmol) was added, and the reaction system was refluxed with water for 3 hours through a water separator, cooled to room temperature, and depressurized The solvent was evaporated to obtain a crude product, which ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com