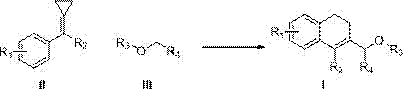
Method for carrying out reaction on methylenecyclopropane derivative and ether compound C(sp<3>)-H bond
A technology of methylene cyclopropane and ether compounds, which is applied in the field of C-H bond reaction between methylene cyclopropane derivatives and ether compounds, achieving the effect of high atom economy, high yield, high efficiency and environmental protection of the reaction system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-16
[0034] Embodiment 1-16 Reaction condition optimization
[0035] Using the compound shown in Formula II-1 and tetrahydrofuran as the reaction raw materials, the influence of various conditions on the reaction (Formula 2) was explored, and representative examples 1-17 were selected. The results are shown in Table 1. Show:
[0036] (Formula 2)
[0037] Wherein the operation of embodiment 1 is as follows:
[0038] Add a magnetic stirrer bar into a 25 mL SCHELNK sealed reaction tube, and add 0.3 mmol (about 70.8 mg) of substrate II-1, then replace the air in the reaction tube with nitrogen for 3-5 times, after Add 2.0 equiv TBHP (tert-butanol peroxide, 5.0 M in decane) (about 0.15 mL) and 2.0 mL tetrahydrofuran under a nitrogen gas flow environment, cover the reaction tube, and place it in an oil bath at 110°C for 24 hours to react. After the reaction was complete, the solvent was evaporated to dryness with a rotary evaporator, and the product was separated and purified with a...
Embodiment 18
[0052] (Formula 3)
[0053] Add a magnetic stirring bar to a 25 mL SCHELNK sealed reaction tube, and put in 0.3 mmol of substrate II-2, then replace the air in the reaction tube with nitrogen, replace it 3-5 times, and then add 2.0 equiv TBHP (tert-butanol peroxide, 5.0 M in decane) (about 0.15 mL) and 2.0 mL tetrahydrofuran, cap the reaction tube, and place it in an oil bath at 110 degrees Celsius to heat for 24 hours. After the reaction was complete, the solvent was evaporated to dryness with a rotary evaporator, and the product was separated and purified with a chromatographic column. The mobile phase was petroleum ether and ethyl acetate, and the ratio was 30:1. Can obtain product 1-2, about 70% of productive rate ( 1 HNMR (400 MHz, CDCl 3 ): 7.42-7.33 (m, 3H), 7.23-7.07 (m, 4H), 7.01 (t, J = 7.2Hz, 1H), 6.57 (d, J = 7.6 Hz, 1H), 4.34 (t, J = 8.0 Hz, 1H), 3.94-3.89 (m,1H), 3.75-3.70 (m, 1H), 2.99-2.81 (m, 2H), 2.61-2.54 (m, 1H), 2.37-2.29 (m,1H ), 1.94-1.64 (m, ...
Embodiment 19
[0055] (Formula 4)
[0056]Add a magnetic stirrer bar into a 25 mL SCHELNK sealed reaction tube, and put in 0.3 mmol of substrate II-3, then replace the air in the reaction tube with nitrogen, replace it 3-5 times, and then add 2.0 equiv TBHP (tert-butanol peroxide, 5.0 M in decane) (about 0.15 mL) and 2.0 mL tetrahydrofuran, cap the reaction tube, and place it in an oil bath at 110 degrees Celsius to heat for 24 hours. After the reaction was complete, the solvent was evaporated to dryness with a rotary evaporator, and the product was separated and purified with a chromatographic column. The mobile phase was petroleum ether and ethyl acetate, and the ratio was 30:1. Product I-3 can be obtained, and the yield is about 68% ( 1 HNMR (400 MHz, CDCl 3 ): 6.84 (s, 1H), 6.72 (s, 1H), 5.94 (s, 2H), 4.46 (t, J =7.2 Hz, 1H), 4.02-3.96 (m, 1H), 3.90-3.84 (m, 1H), 2.77-2.71 (m, 2H), 2.28-2.16 (m, 2H), 2.12-2.05 (m, 1H ), 1.99-1.94 (m, 2H), 1.76-1.70 (m, 1H); 13 C NMR (100 MHz, CDC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com