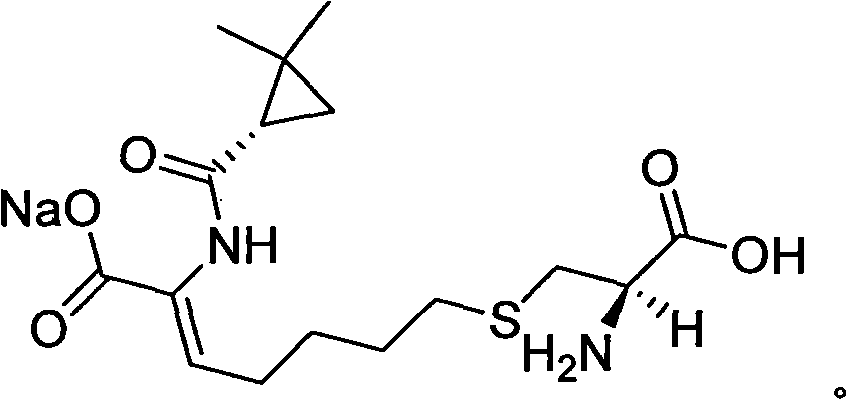
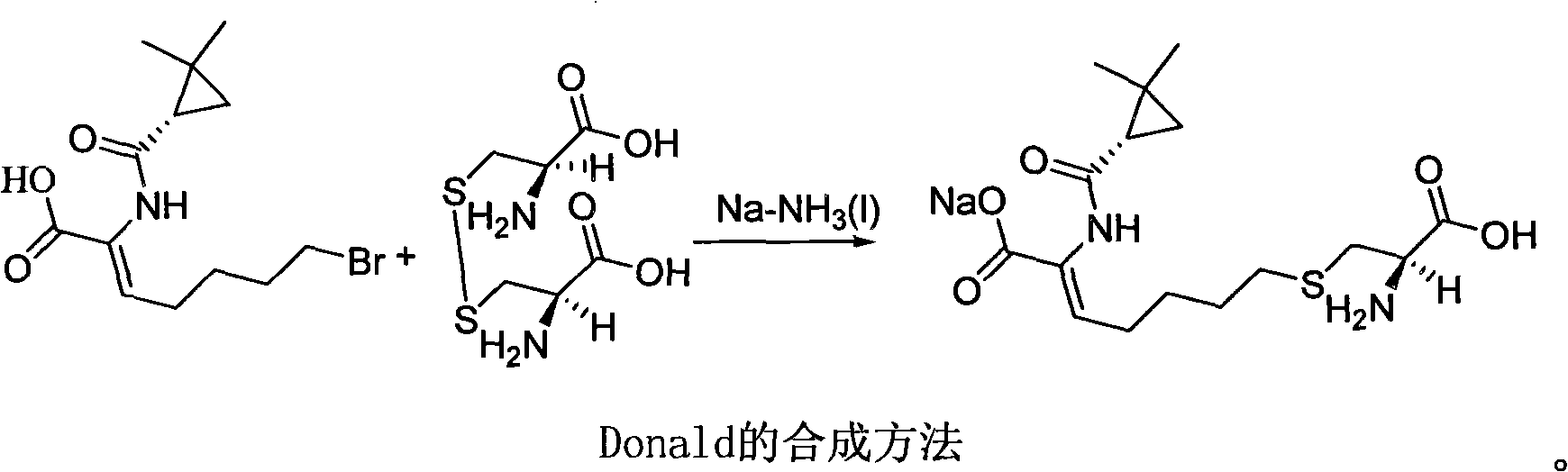
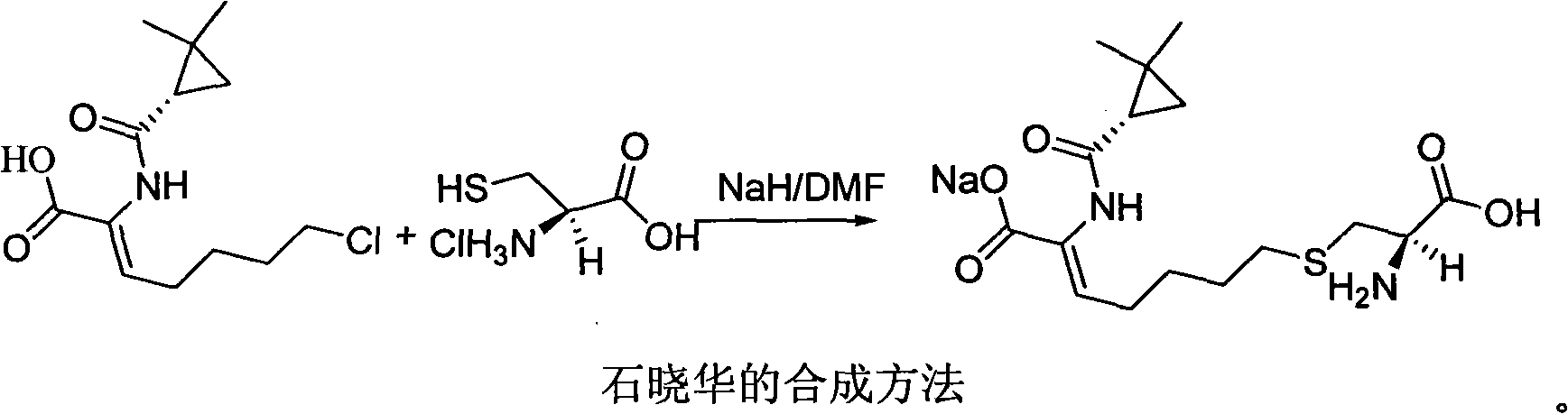
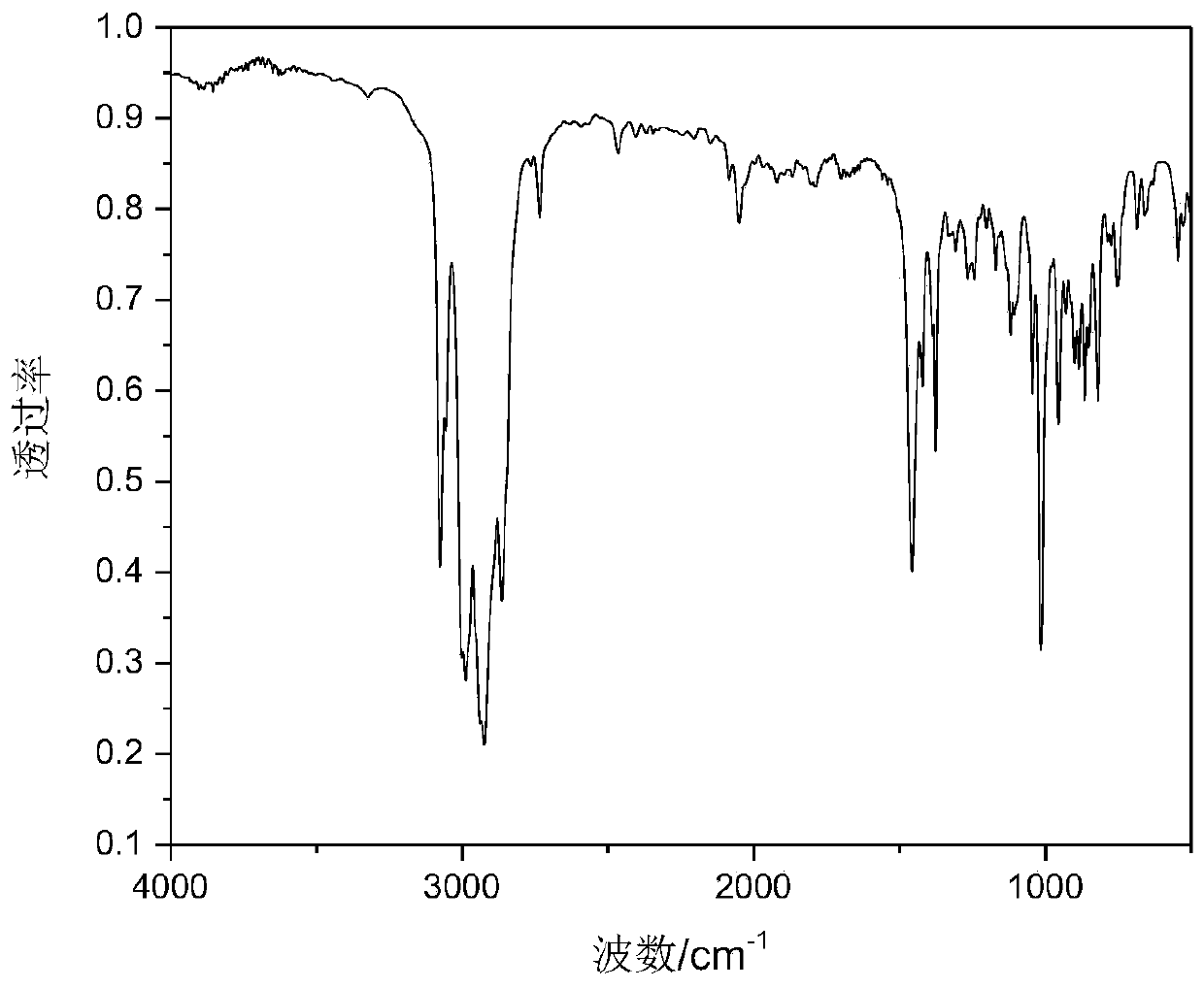
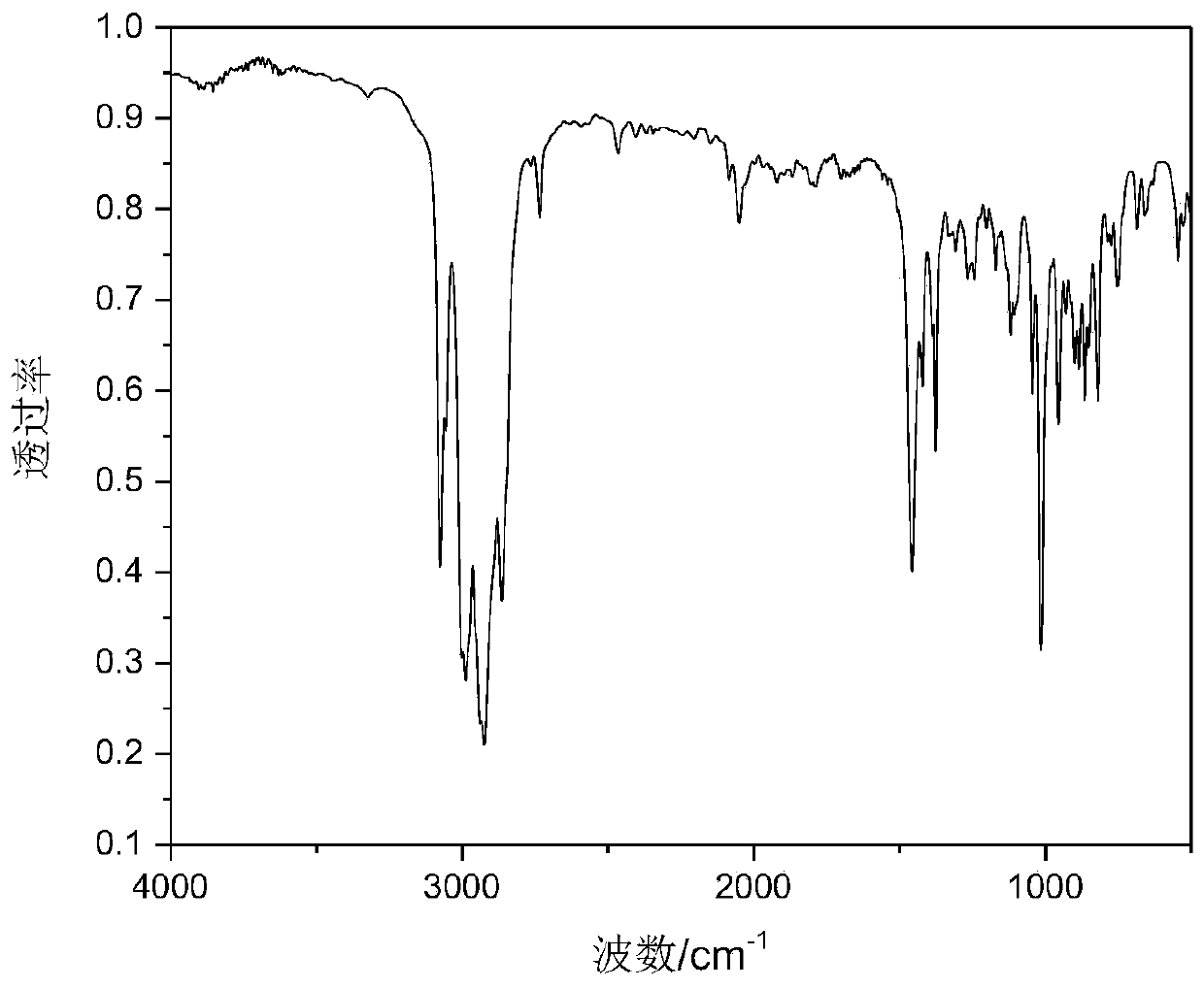
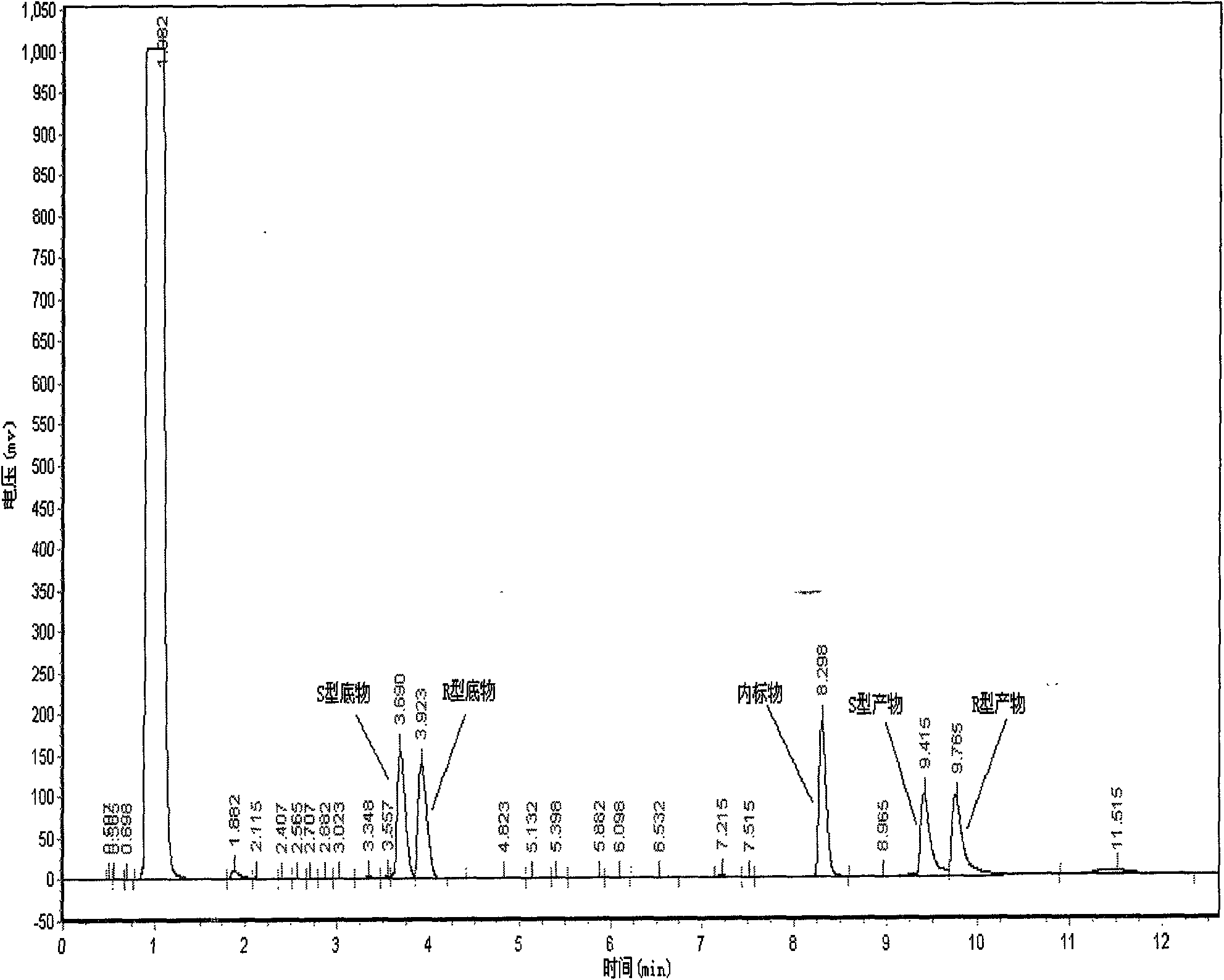
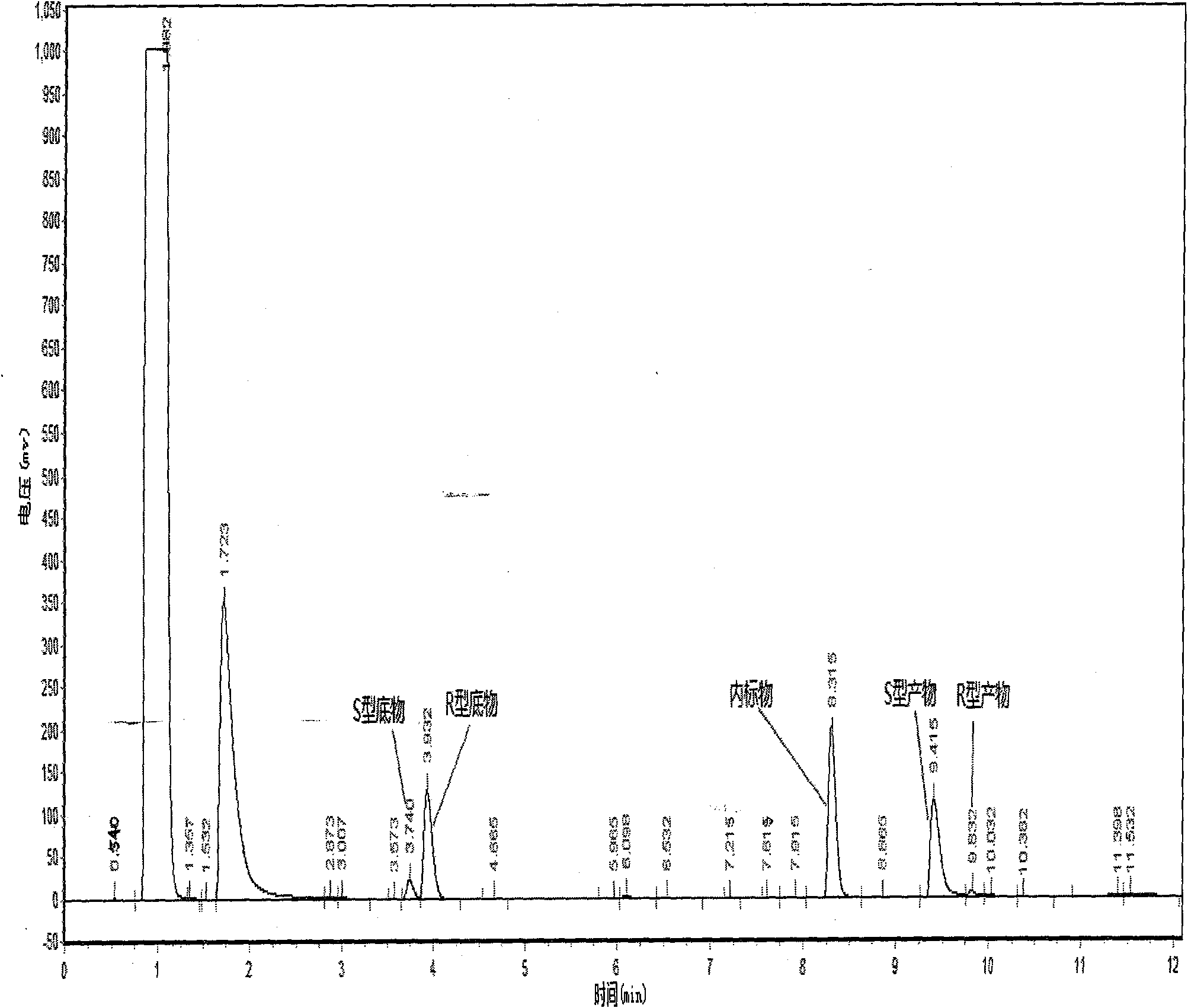
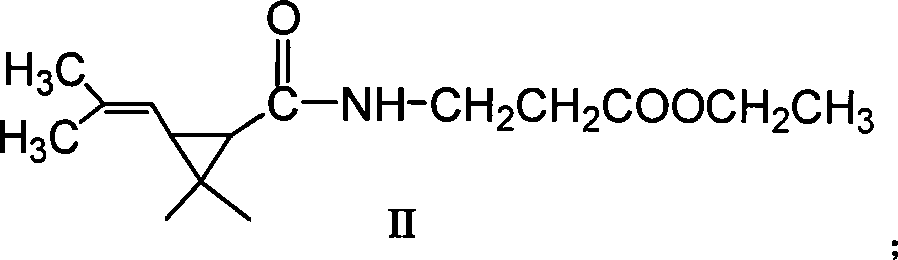
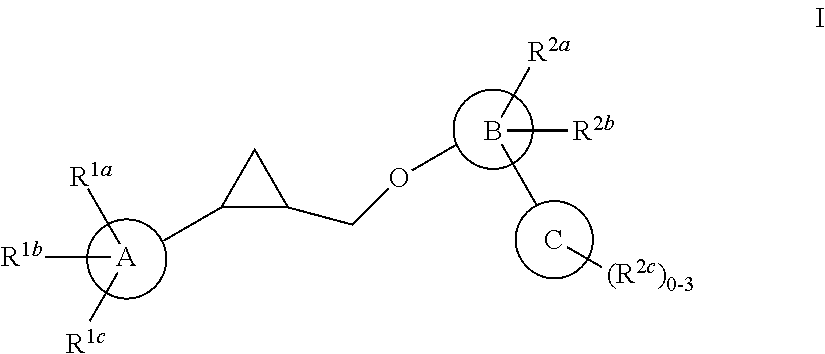
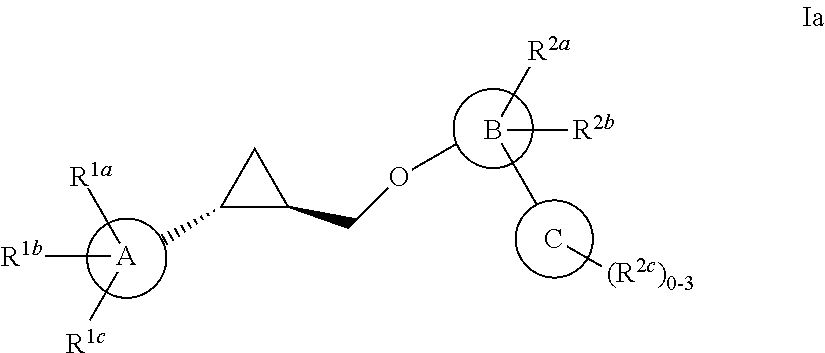
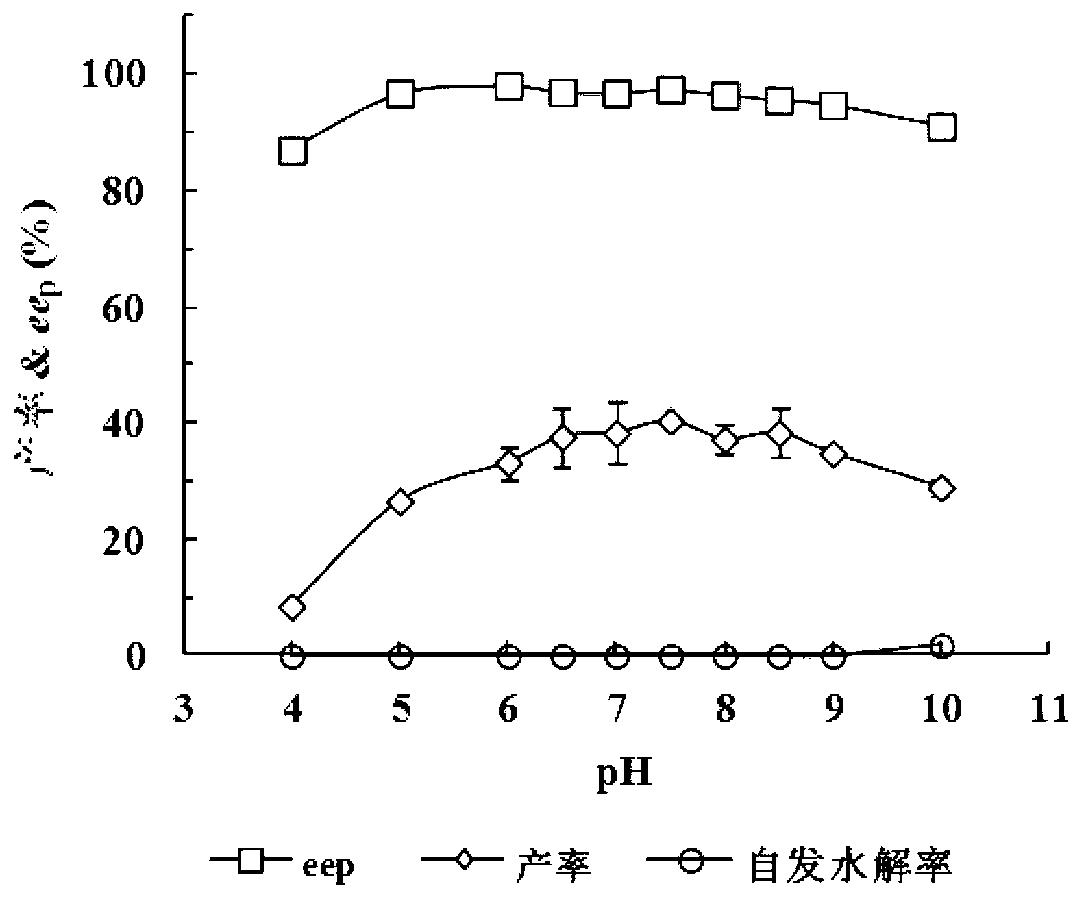
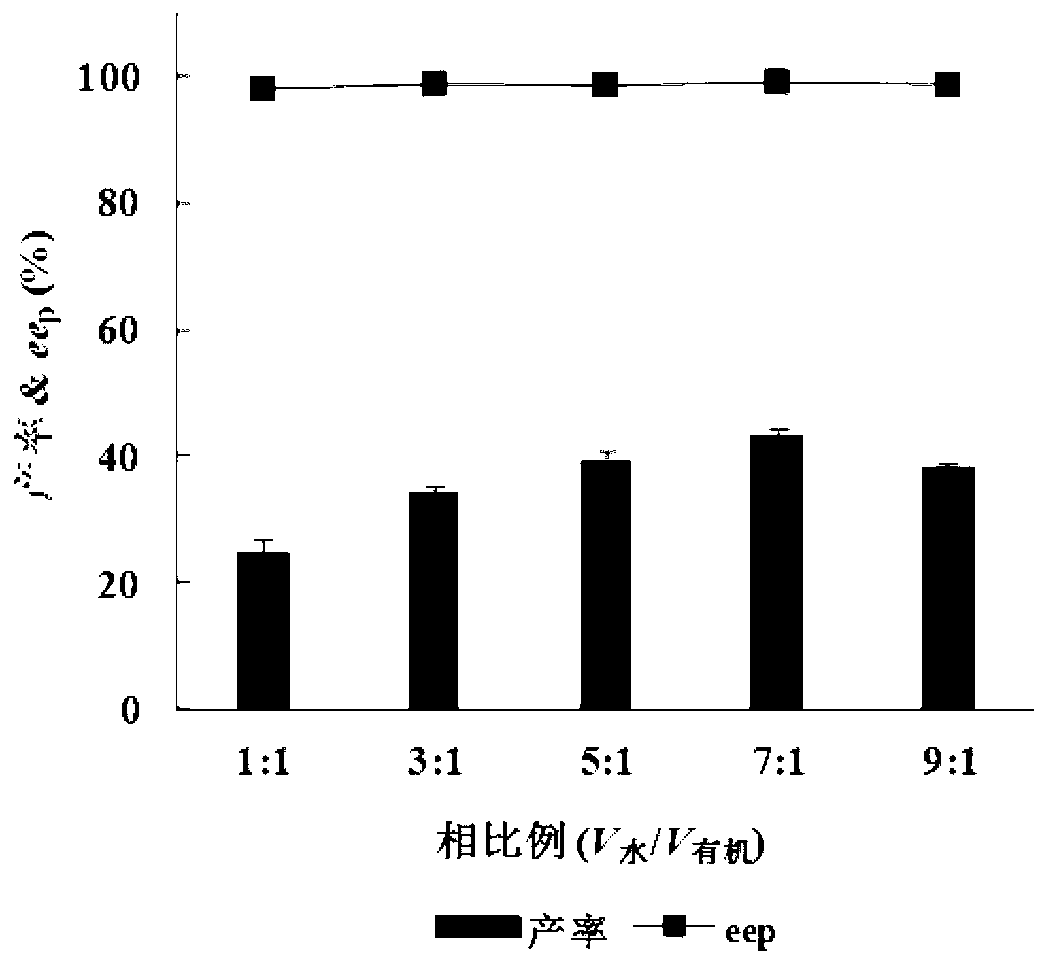
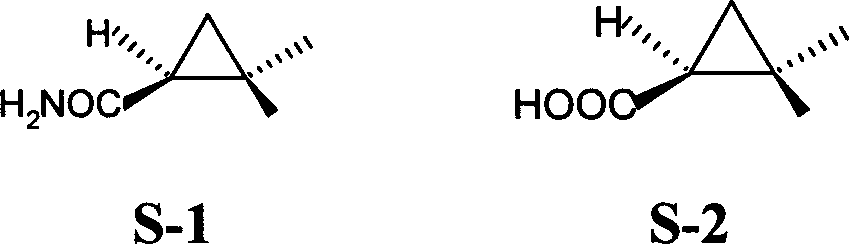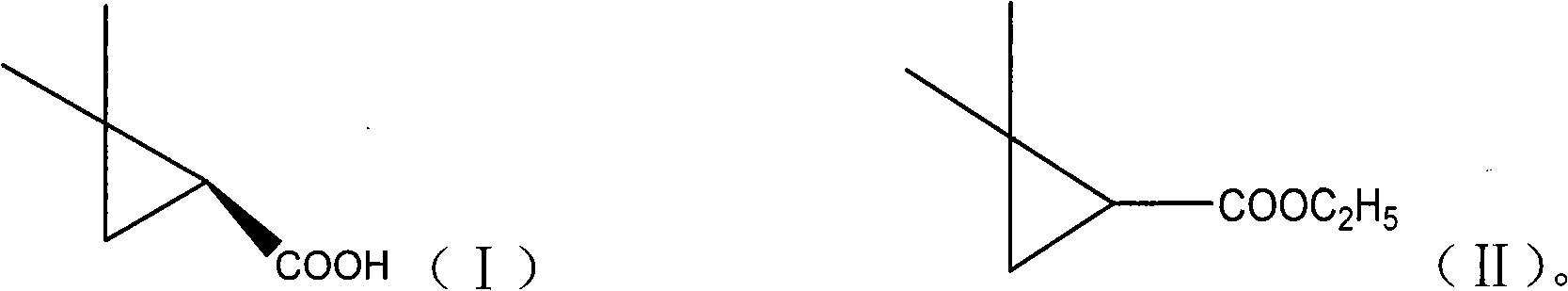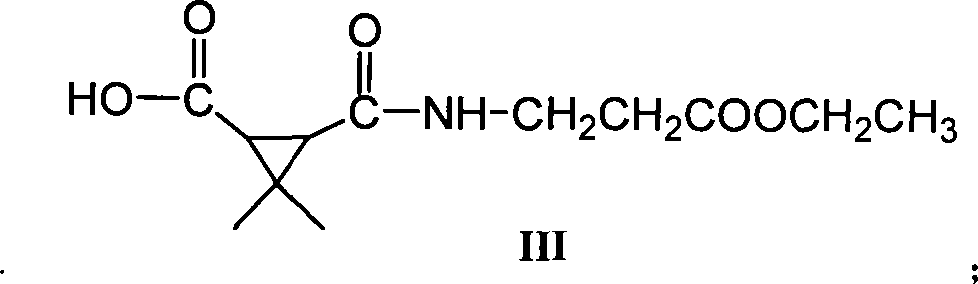Patents
Literature
154 results about "Methylcyclopropane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
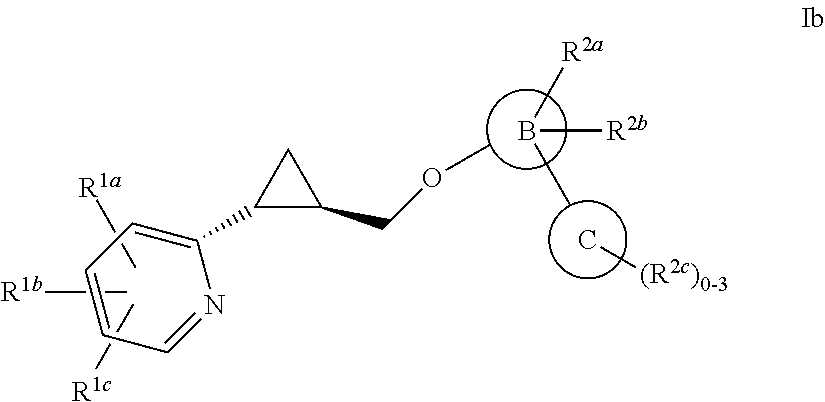
Methylcyclopropane is an organic compound with the structural formula C₃H₅CH₃. This colorless gas is the monomethyl derivative of cyclopropane.
Insecticidal composition
ActiveCN101473842AGood effect of killing mosquitoesBiocideAnimal repellantsCarboxylic acidInsect pest
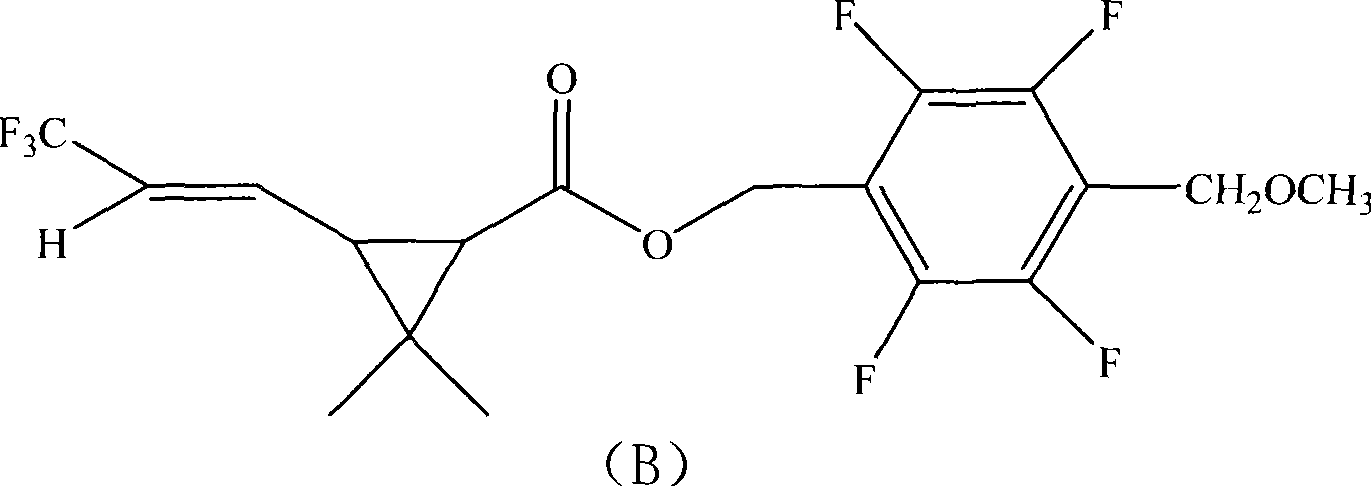
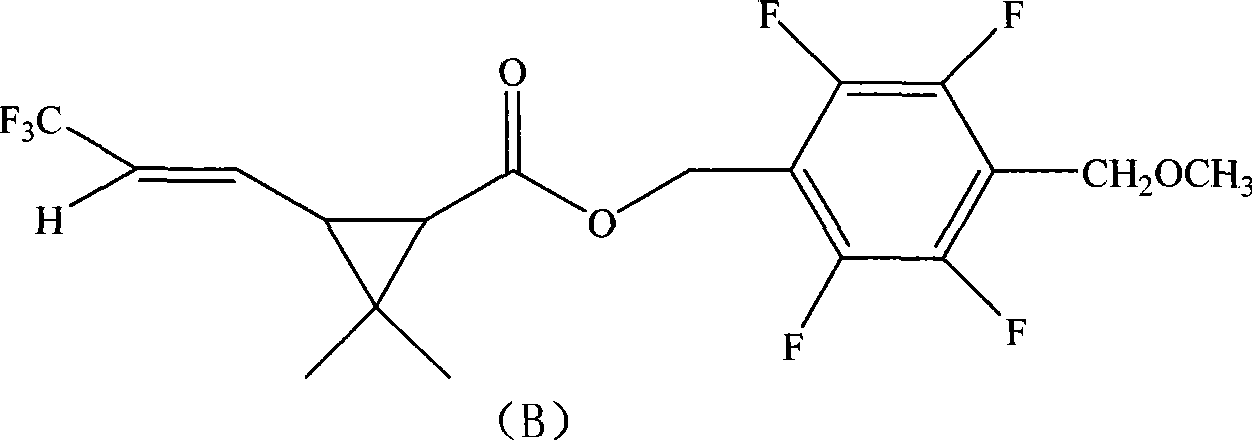
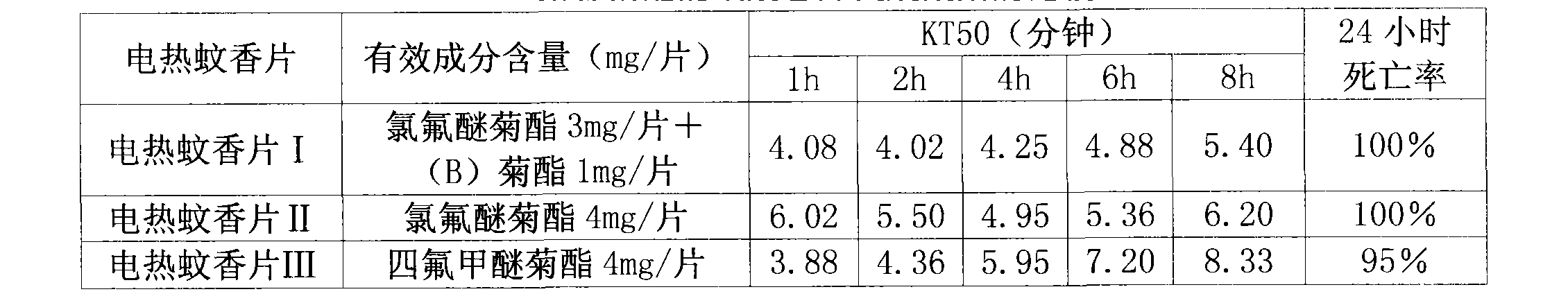
The invention provides an insecticidal composition for controlling the sanitary insect pests such as mosquitoes, flies, blattella germanica, and the like. The insecticidal composition comprises a component (A) dichlor dimefluthrin and a component (B) 2, 3, 5, 6-tetrafluoro-4-methoxyl methyl benzyl-3-(3, 3, 3-trifluoro-propenyl)-2, 2-dimethyl propane carboxylate, wherein, the mass ratio between the component (A) and the component (B) is 1:20 to 99:1. The coil type mosquito repellent incenses, the electric mosquito-repellent mats, the electric mosquito repellent vaporizer, the insecticide aerosol and other products which are prepared by using the insecticidal composition have the advantages of rapid killing to the insect pests and long-term drug effect.
Owner:JIANGSU YANGNONG CHEM +1
1-methyl-3-(2-methylcyclopropane)-1-cyclopropene inclusion complex for keeping fruits, vegetables and flowers fresh and preparation method thereof
InactiveCN101990937AExtend your lifeImprove stabilityDead plant preservationFruit and vegetables preservationBiotechnologyAdditive ingredient
The invention discloses a 1-methyl-3-(2-methylcyclopropane)-1-cyclopropene stable inclusion complex for keeping fruits, vegetables and flowers fresh, which is characterized in that: the inclusion complex is formed by reaction of 1-methyl-3-(2-methylcyclopropane)-1-cyclopropene and cyclodextrin. The inclusion complex is a solid fruit, vegetable and flower fresh-keeping agent material. The 1-methyl-3-(2-methylcyclopropane)-1-cyclopropene gas serving as an effective ingredient in the inclusion complex accounts for 0.001 to 4.5 weight percent of the fresh-keeping agent. The inclusion complex is mainly used for keeping the fruits, vegetables and flowers with climacteric after-ripening aging fresh, and particularly has predominant fresh-keeping effect on leaf vegetables and fruits. The inclusion complex has the advantages of high effective ingredient, difficult oxidation, convenience for use and low cost, and is easy for storage and transportation; and the preparation method is performed at normal pressure, has the advantages of simple process, safety, reliability, high product yield and low cost, and is easy for implementation.
Owner:XI AN JIAOTONG UNIV
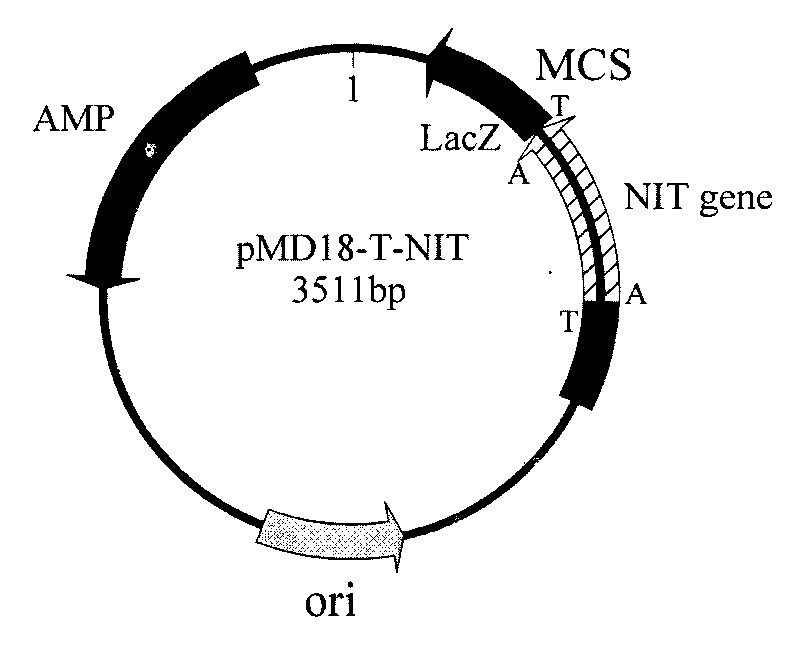
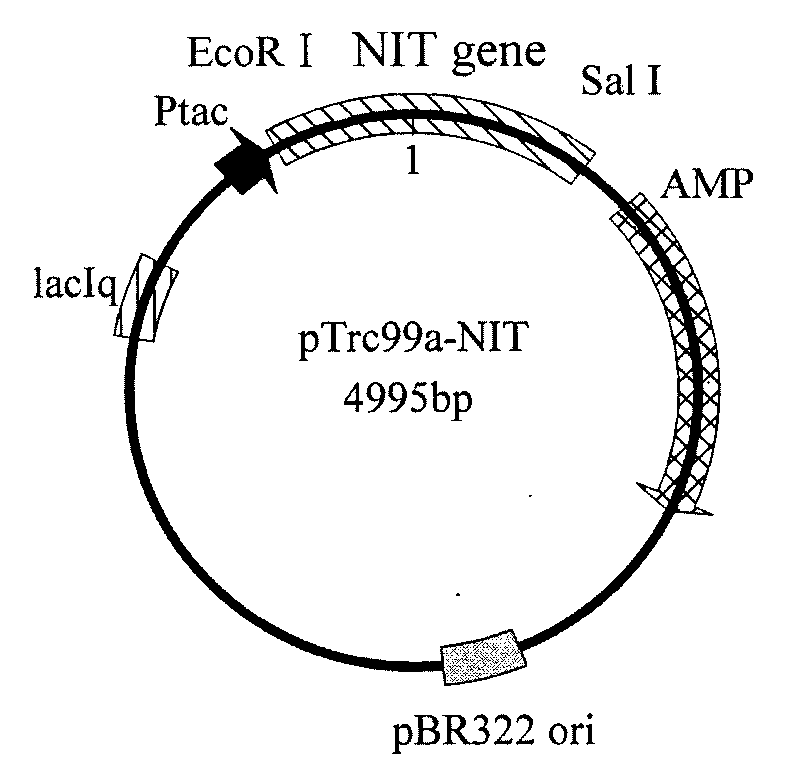
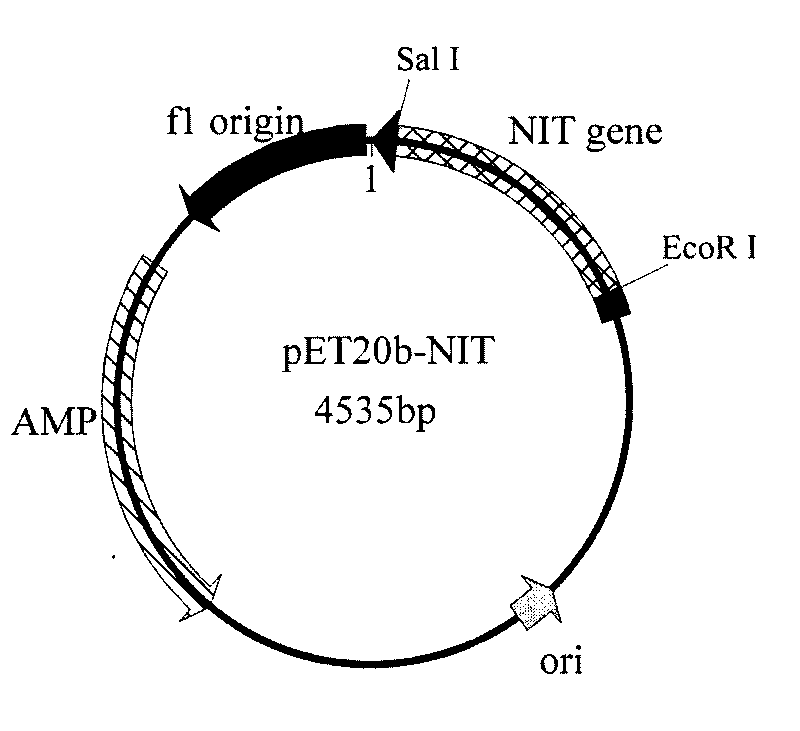
Nitrilase gene, vector, engineering bacteria and application thereof
The invention provides a nitrilase gene coding nitrilase, a recombinant vector containing the gene, a recombinant gene engineering bacteria obtained by converting the recombinant vector and application thereof in preparing recombinant nitrilase. The nitrilase gene can be connected with an expression vector for construction to obtain endoenzyme expression recombinant plasmid containing the gene or secretion expression recombinant plasmid, and then the endoenzyme expression recombinant plasmid containing the gene or the secretion expression recombinant plasmid is respectively and correspondingly converted to a colibacillus bacterial strain to obtain recombinant colibacillus; the recombinant colibacillus contains recombinant nitrilase and can recombine colibacillus into an enzyme resource for biological catalysis and conversion. The recombinant nitrilase serves as the enzyme for conversion, and racemisation mandelonitrile, acrylonitrile, iminodiacetonitrile or 2,2-dimethylcyclopropane carbonitrile and the like serve as a substrate for converting to react and prepare corresponding R-mandelic acid, crylic acid, iminodiacetic acid or chiral 2,2-dimethylcyclopropane formic acid and the like.
Owner:ZHEJIANG UNIV OF TECH
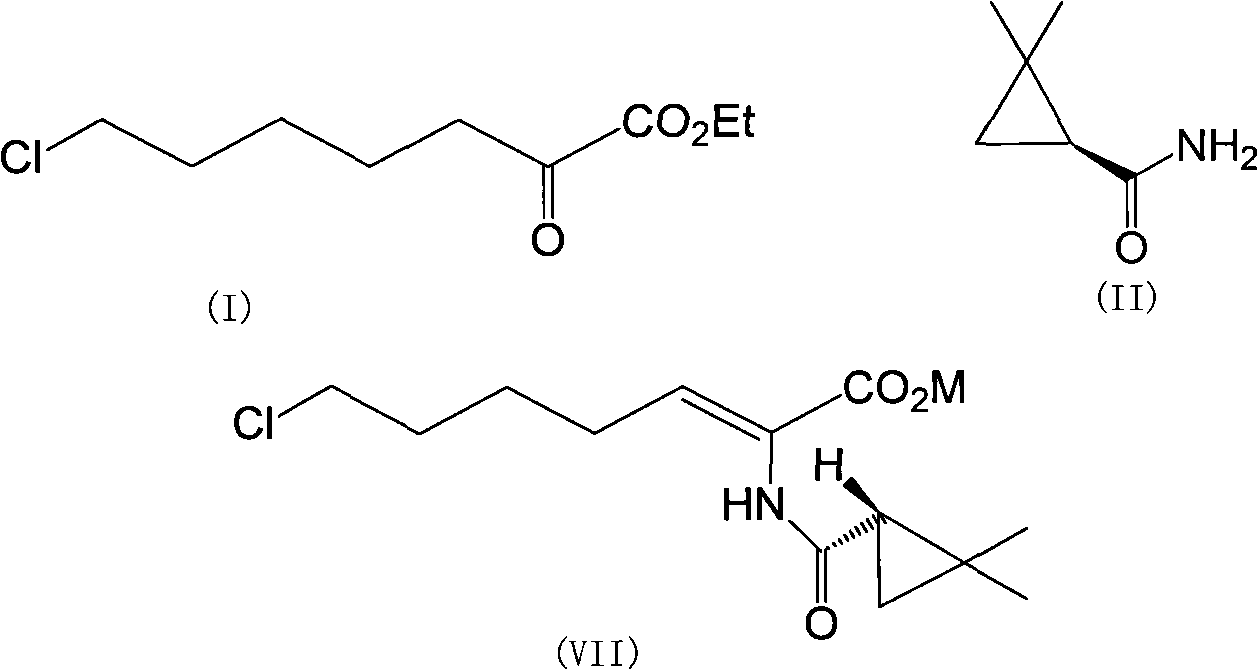
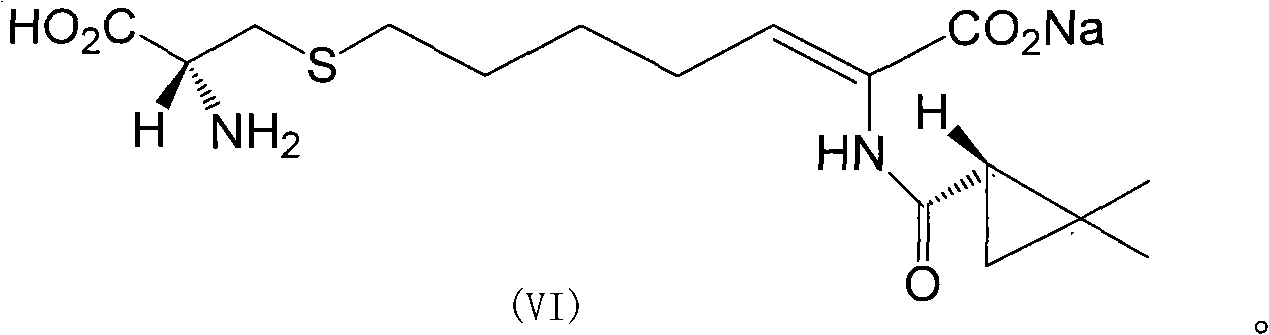
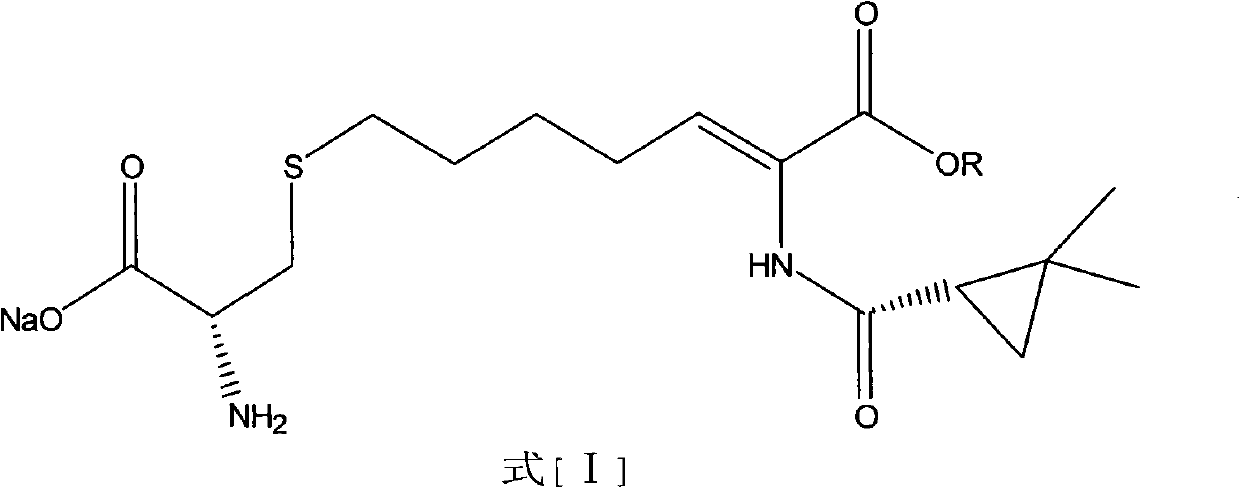
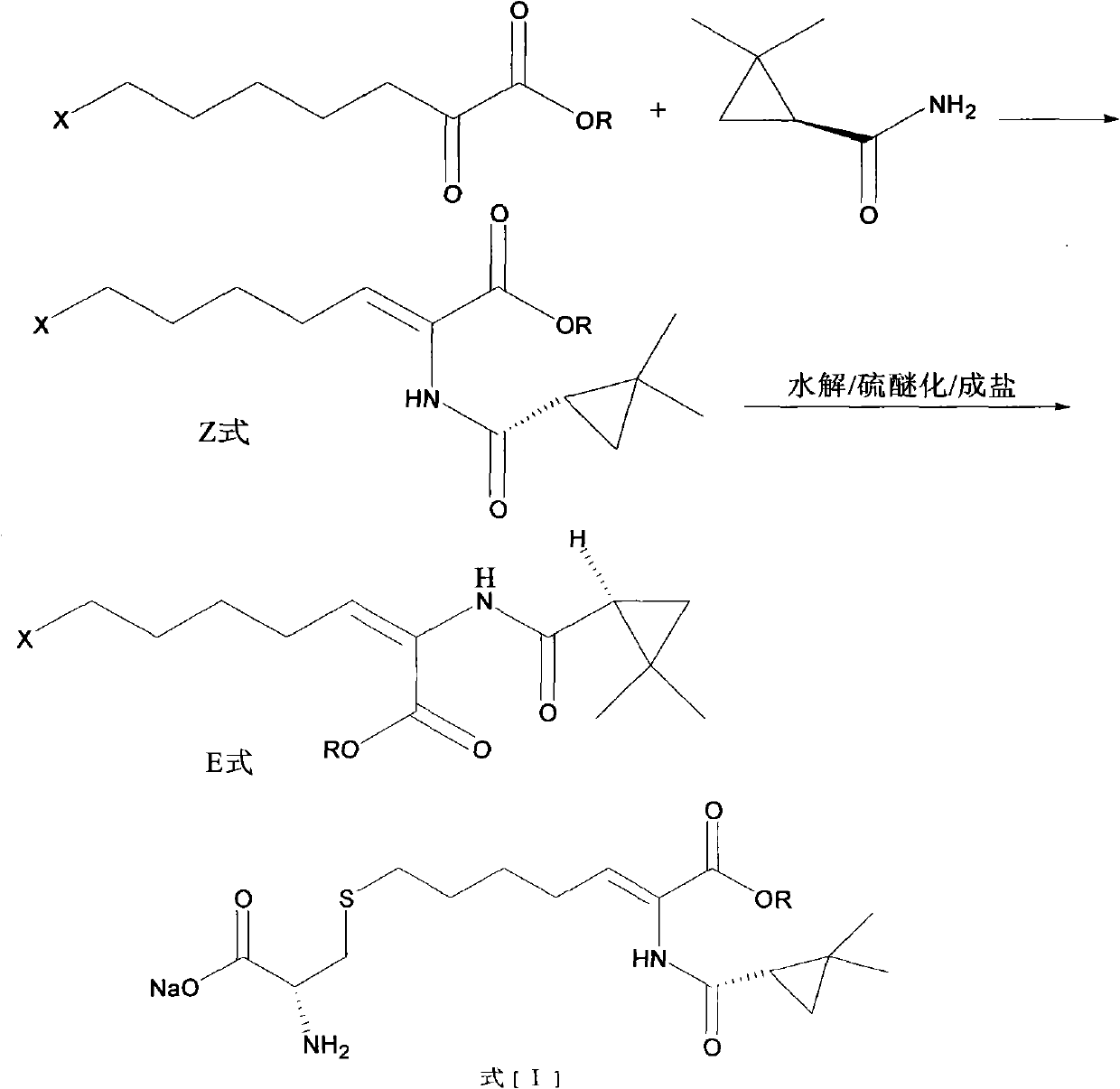
Preparation method of cilastatin sodium
InactiveCN101792410ASolve the pollution problemEasy to solveSulfide preparationSodium hydroxideChrominance
The invention discloses a preparation method of cilastatin sodium, comprising the following steps of: taking 7-chloro-oxo-ethyl oenanthate and (S)-(+)-2, 2-dimethyl cyclopropane formamide as raw materials, and carrying out condensation reaction and basic hydrolysis to obtain (Z)-7-chloro-2((S)-2, 2-dimethyl cyclopropane formamido)-2-heptenoic acid; then, adding alkaline compound for reaction, and separating and purifying to obtain (Z)-7-chloro-2((S)-2, 2-dimethyl cyclopropane formamido)-2-heptenoic acid metal salt crystal; leading the metal salt crystal and cysteine hydrochloride for condensation reaction, acidizing the reaction liquid by hydrochloric acid, washing by organic solvent and concentrating, then loading sample into macroporous absorption resin for purifying, directly using sodium hydroxide solution for elution, and obtaining cilastatin sodium solid after the treatment of eluent. The preparation method simplifies the preparation technique, and effectively improves the chrominance, the purity and the yield of the cilastatin sodium.
Owner:ZHEJIANG UNIV OF TECH +1
Anti-mouse agent formula for cable insulation layers
InactiveCN104987663AProblems of preventing food damage from corroding wires and cablesInsulation layerMethylcyclopropane
The invention relates to an anti-mouse agent formula for cable insulation layers. The formula comprises, by mass, 30% of capsaicinoids or more, 30% of o-isopropylphenyl-n-methylcarbamate or alpha-cyano-3-phenoxy-benzyl-d-cis-2 or more, 30% of 2-dimethylcyclopropanecarboxylate or more, 1% of water or less and 5% of epoxy oil or more. Because irritant capsaicinoid extractives obtained from natural plants are adopted, the anti-mouse agent has the advantages of being nontoxic, environmentally friendly and free from traditional highly toxic pesticides, and the situation that PVC or PE wires and cables are seriously bitten or corroded by mice or termites when used in areas where mice or termites are rampant can be avoided.
Owner:FOSHAN YUEJIAXIN WIRE & CABLE
Papermaking sewage treatment agent
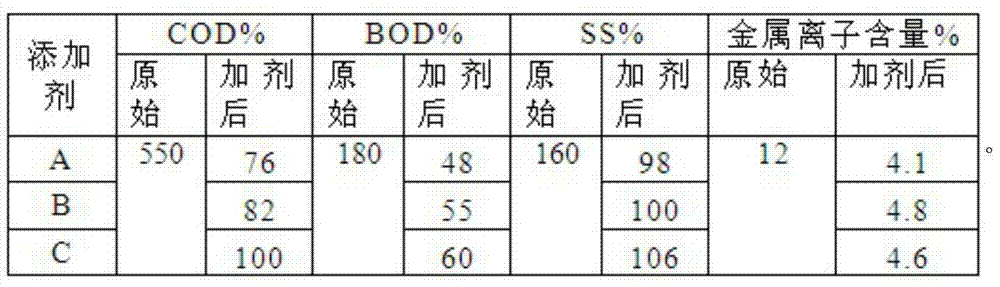
ActiveCN103708568AReduce CODReduce BODWater/sewage treatmentWaste water treatment from plant processingSodium acetatePapermaking
The invention provides a papermaking sewage treatment agent. The papermaking sewage treatment agent comprises dithiocarbamate, ferrous sulfate, citric acid, sodium acetate, polyferric chloride, trimethylcyclopropyl ammonium chloride, chitin and bentonite. The treatment agent allows the physical and chemical indexes of papermaking sewage reach GB18918-2002 standards, and effectively reduces the COD, BOD, SS and the metal ion content in the papermaking sewage.
Owner:珠海横琴森禾生物科技控股有限公司
Preparation method of cilastatin sodium
ActiveCN102702051AReduce generationHigh yieldOrganic compound preparationSulfide preparationIsomerizationCilastatin sodium
The invention belongs to the field of pharmaceutical synthesis, and provides a preparation method of cilastatin sodium, and the method comprises the following steps: performing condensation, alkaline hydrolysis, and thioetherification of raw materials of 7-oxyhalogen alkyl heptylate and (s)-2,2-dimethylcyclopropane methanamide, adjusting the pH value of the thioetherified solution, washing by a nonpolar solvent, performing isomerization, purification by a neutral macroporous adsorption resin, and salt formation so as to obtain the cilastatin sodium solid. The invention reduces the generation of impurities, improves the isomerization efficiency, simplifies the preparation process, and effectively improves the yield and purity of cilastatin sodium.
Owner:SHANDONG NEWTIME PHARMA
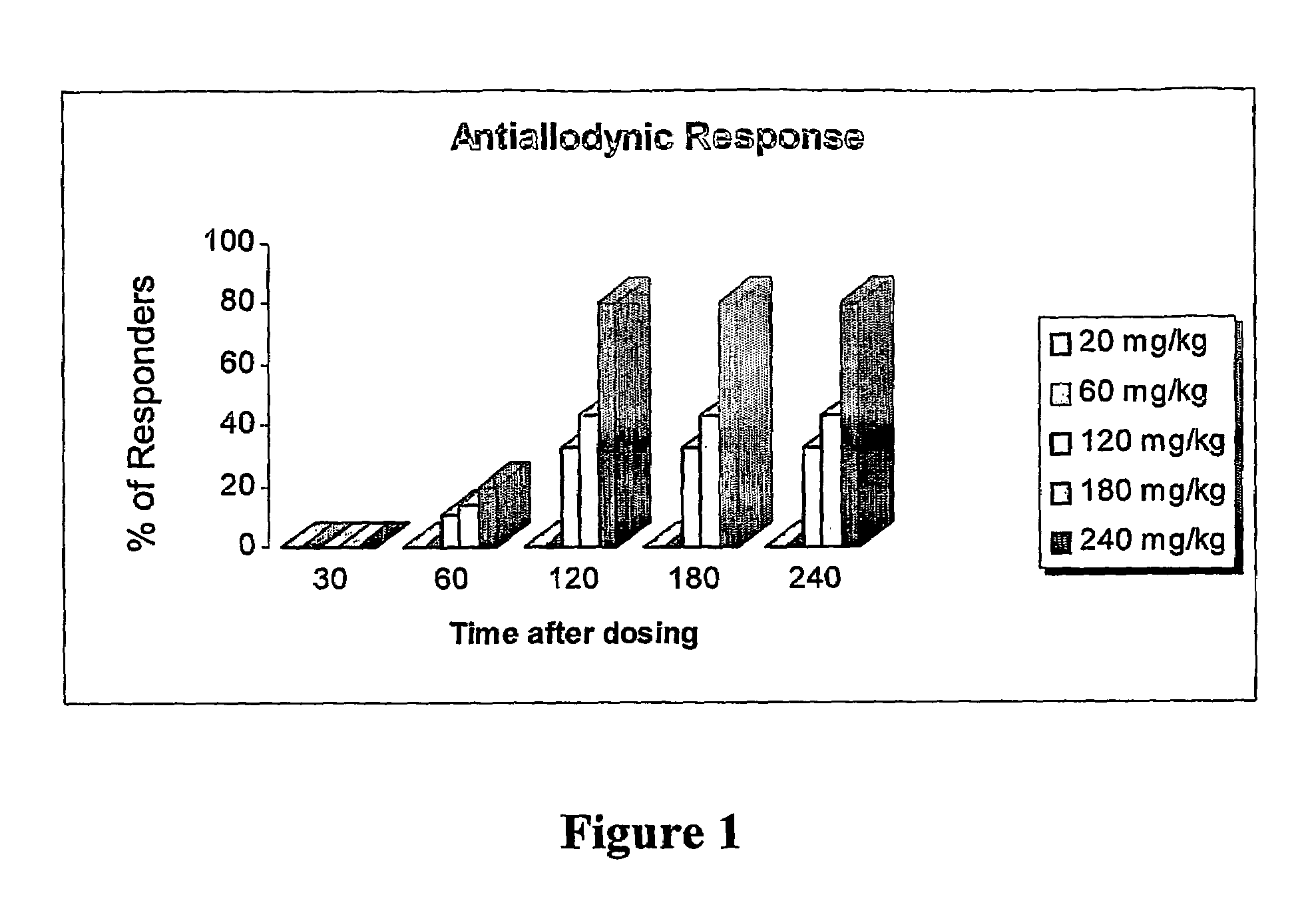
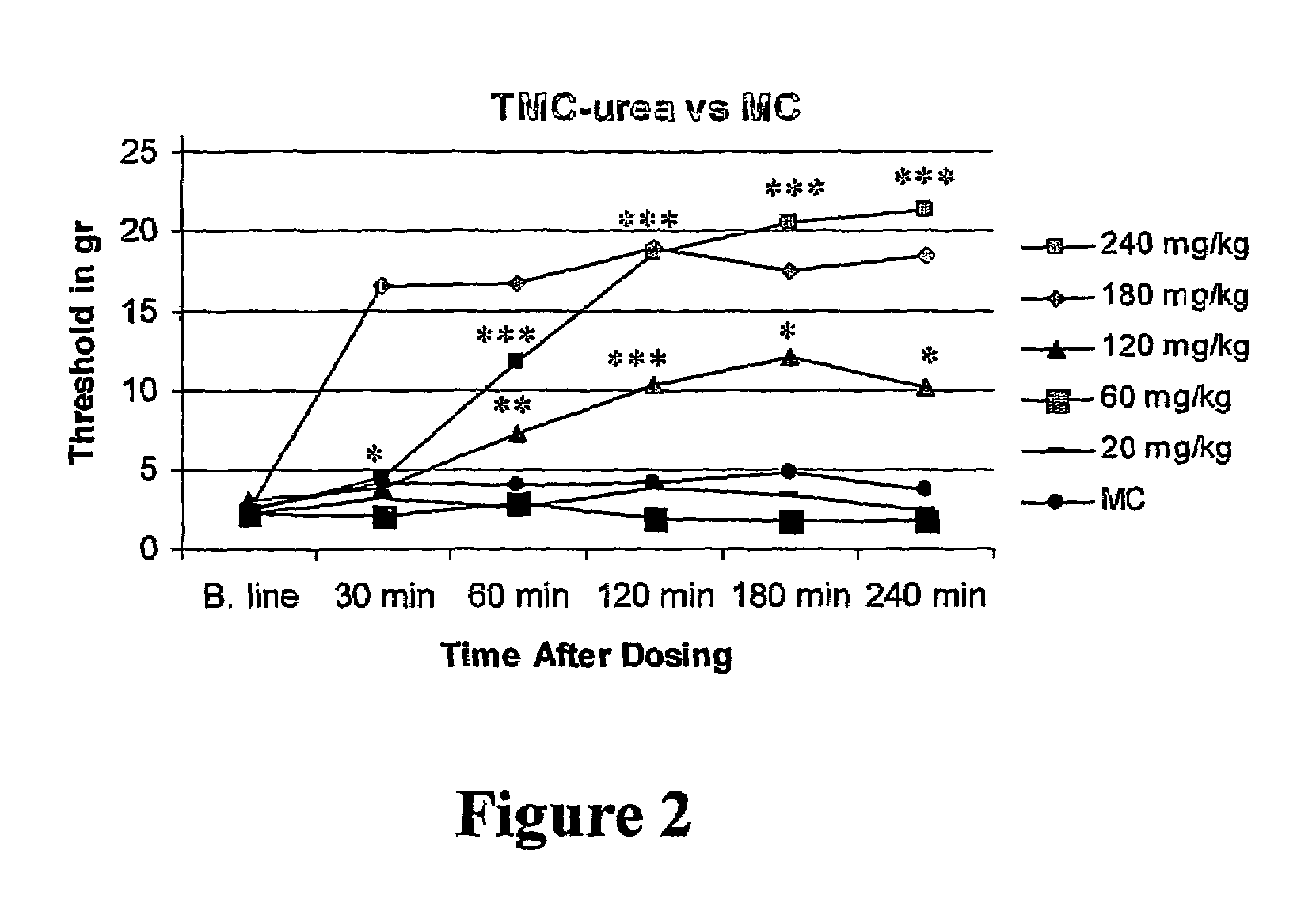
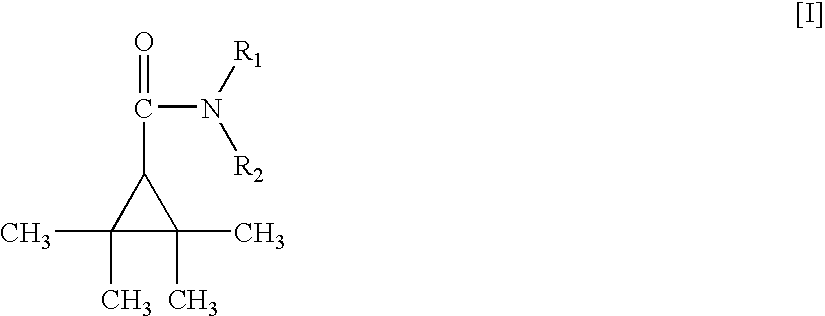
Amide derivatives of 2,2,3,3-tetramethylcyclopropane carboxylic acid
InactiveUS7232929B2Prevent and and ameliorate effectBiocideUrea derivatives preparationDiseaseCarboxylic acid
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Preparation method for roflumilast intermediate
InactiveCN102503815AThe total yield of the five-step reaction is highMild reaction conditionsOrganic compound preparationCarboxylic compound preparationBenzaldehydeRoflumilast
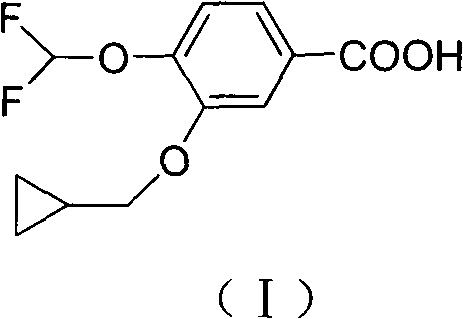
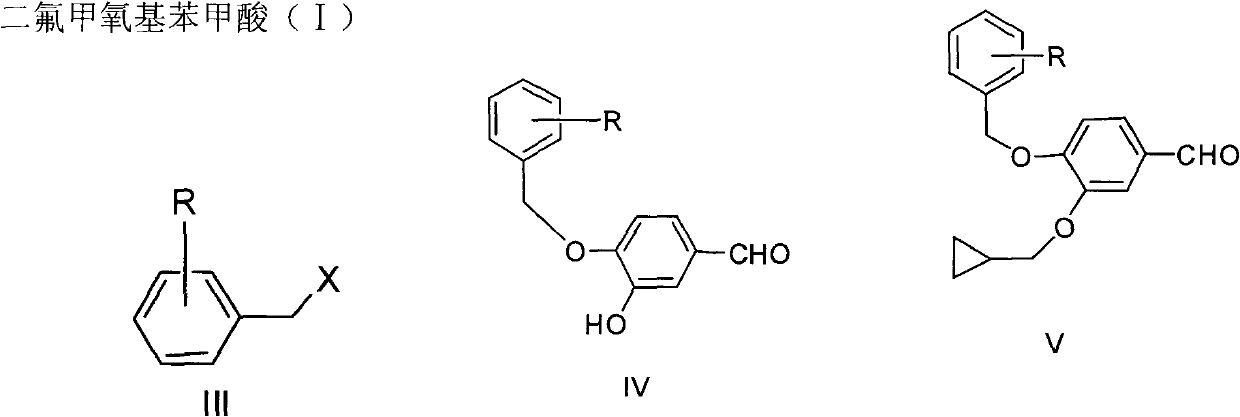
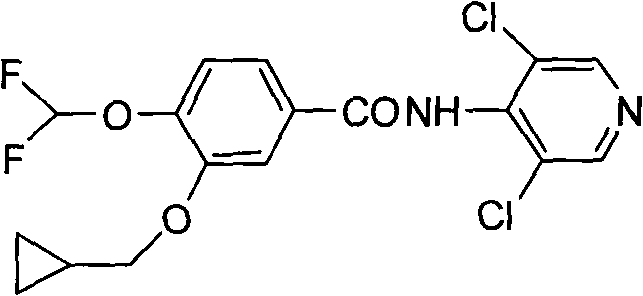
The invention discloses a preparation method for a roflumilast intermediate. The preparation method is characterized by comprising the following steps of: etherifying 3,4-dihydroxy benzaldehyde serving as a raw material and 3-hydroxyl by using benzene ring non-substituted, mono-substituted or poly-substituted benzyl protection 4-hydroxy and halogenated methyl cyclopropane; performing catalytic hydrogenolysis to obtain 4-hydroxy-3-cyclopropyl methoxy-benzaldehyde; etherifying and oxidizing the 4-hydroxy-3-cyclopropyl methoxy-benzaldehyde with difluoromonoch-loromethane to obtain 3-cyclopropyl methoxy-4-difluoromethoxybenzoic acid (I) serving as the roflumilast intermediate. The preparation method has the advantages of easiness and convenience for operating, mild reaction conditions, stable quality, simple post-treatment and no need of complex operation such as column chromatography and the like, and is suitable for industrial production.
Owner:NANJING TIANHAI MEDICAL TECH
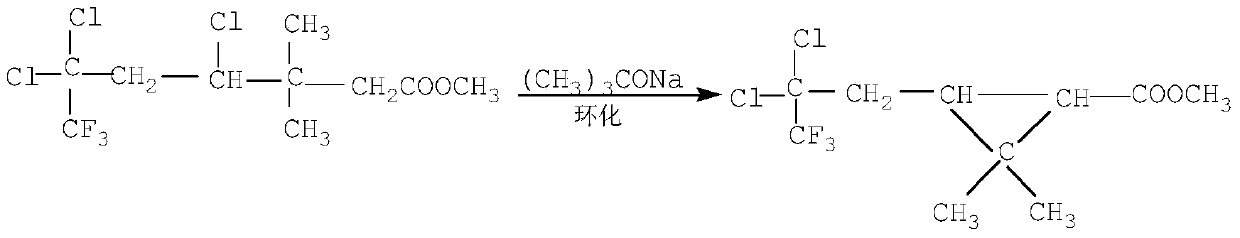
Continuous production method for trifluoro monochloro chrysanthemic acid
InactiveCN105503582ABest production methodQuality improvementOxygen-containing compound preparationOrganic compound preparation4-pentenoic acidChrysanthemic acid
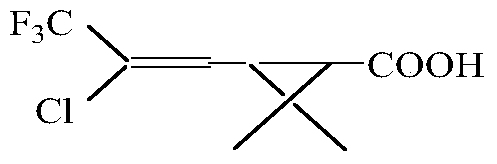
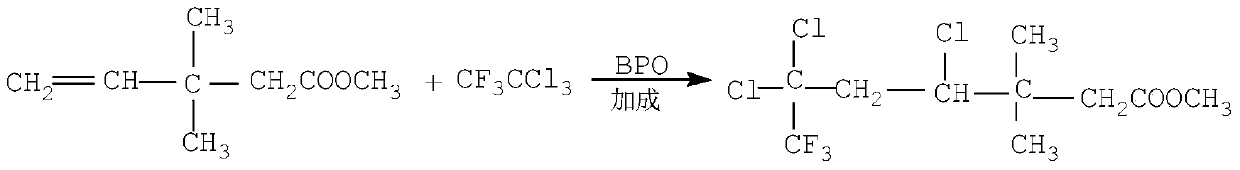
The invention discloses a continuous production method for trifluoro monochloro chrysanthemic acid. The method includes the steps of (1) performing an addition reaction to prepare 3,3-dimethyl-4,6,6-trichloro-7,7,7-trifluoro heptylic acid ester with 3,3-dimethyl-4-pentenoic acid methyl ester and trifluorotrichloroethane as initial raw materials; (2) performing a cyclization reaction with sodium tert-butoxide to generate cis(trans)-3-(2,2-dichloro-3,3,3-trifluoropropyl)-2,2-dimethyl cyclocarboxylate; and (3) performing a saponification reaction to prepare 3-(2-chloro-3,3,3-trifluoro-propylene-1-yl)-2,2-dimethylcyclopropane carboxylic acid, namely, the trifluoro monochloro chrysanthemic acid. In the invention, continuous control is employed in the production method, so that the method is simple in process, is high in equipment utilization, is high in yield and stable in quality of products, is low in production cost and has a wide application prospect.
Owner:LIANYUNGANG CCA CHEM CO LTD
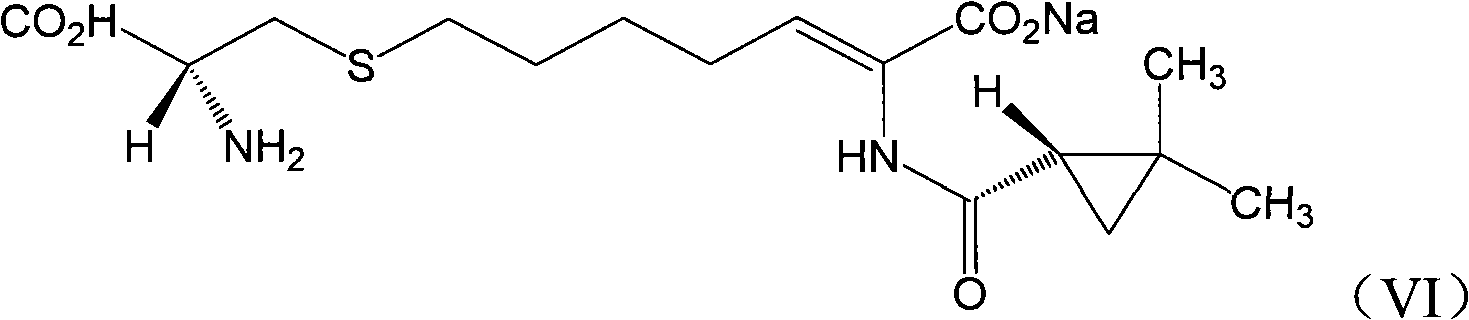
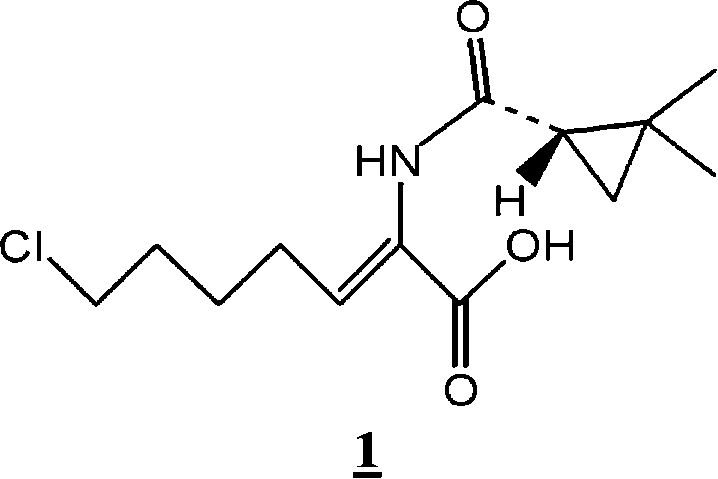
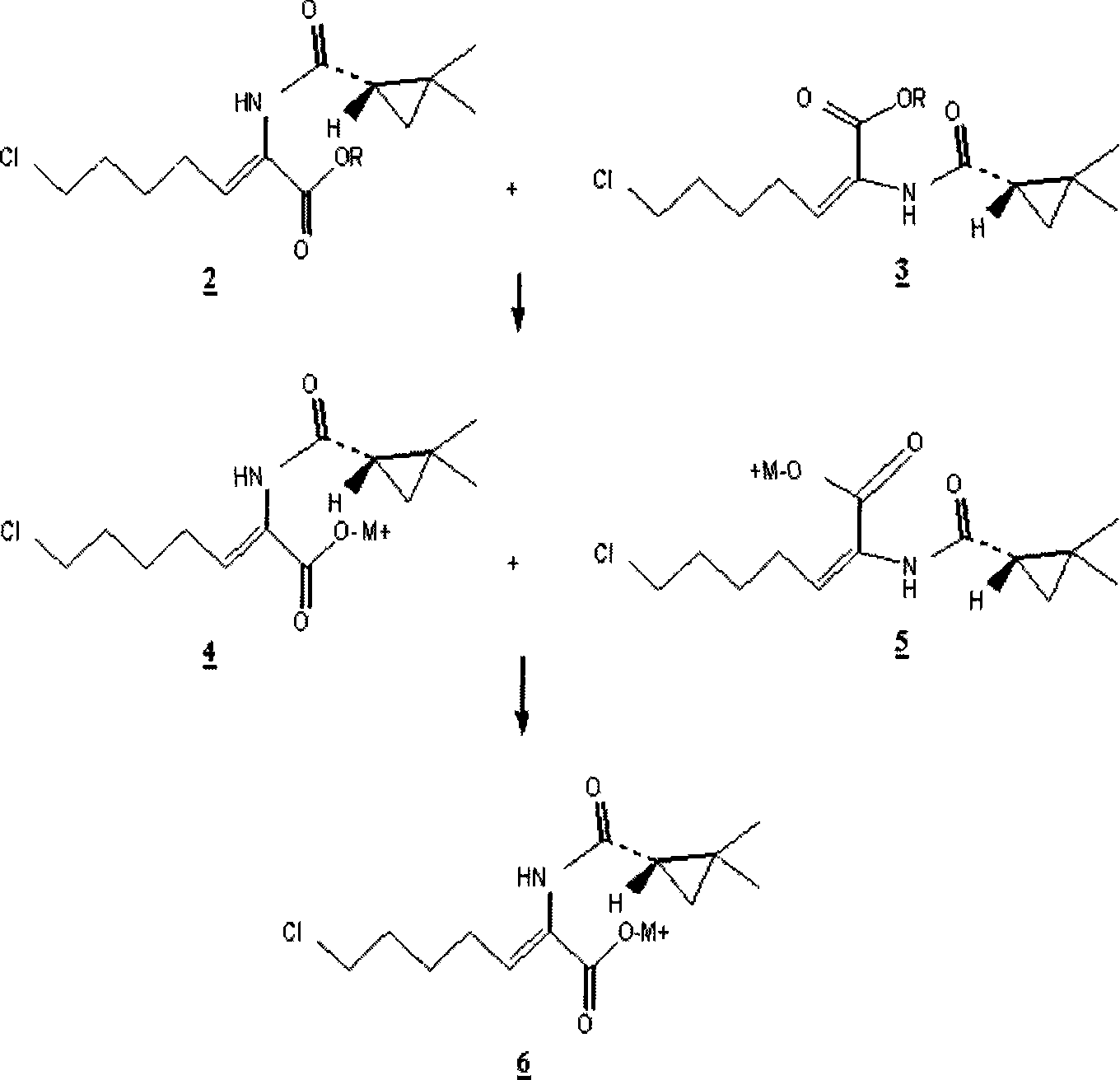
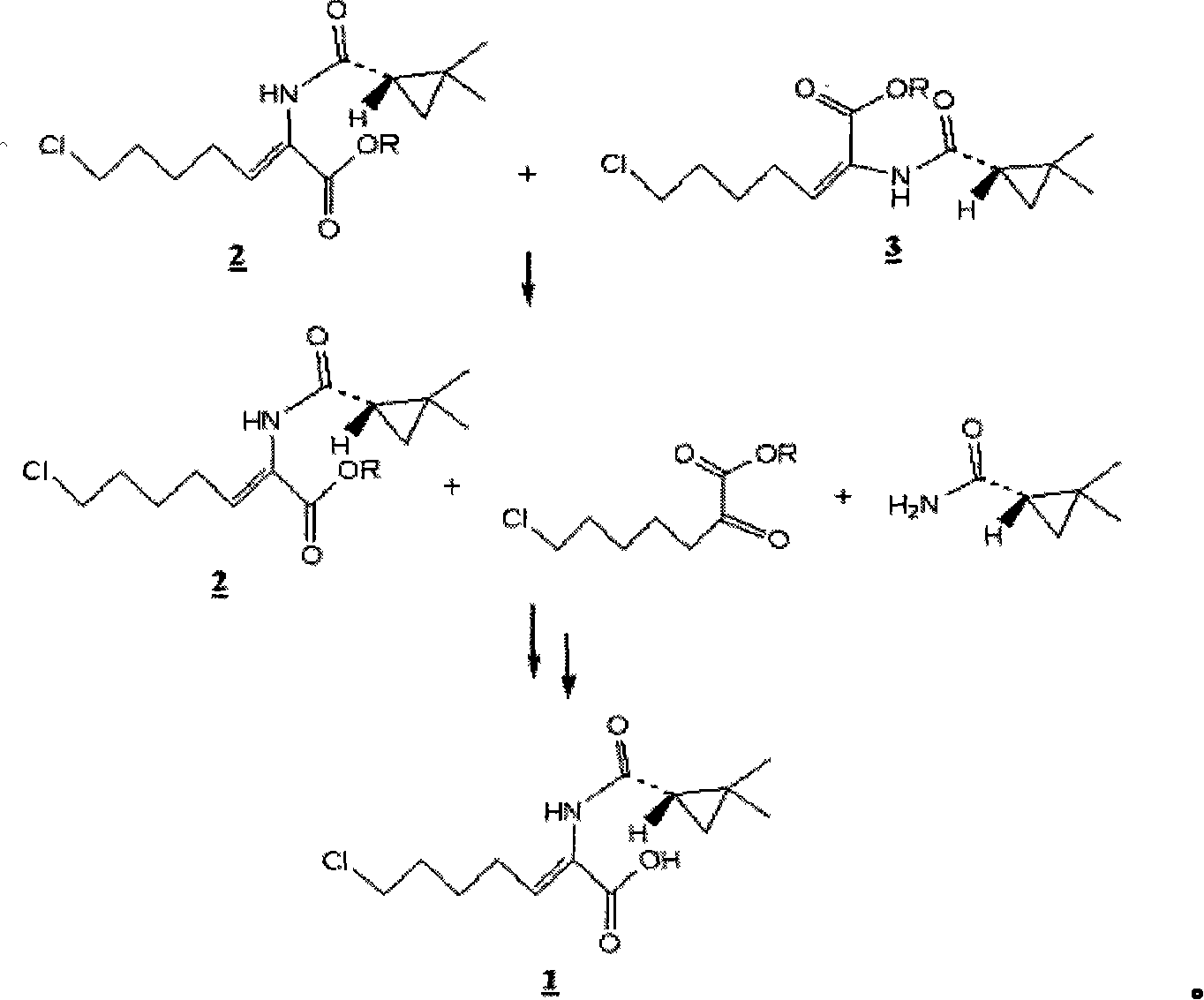
Preparation method for (Z)-7-chloro-((S)-2,2-dimethylcyclopropanecarboxamido)-2-heptenoic acid
InactiveCN101200434AMolecular sieve catalystCarboxylic acid amides optical isomer preparationHydrolysisMethylcyclopropane
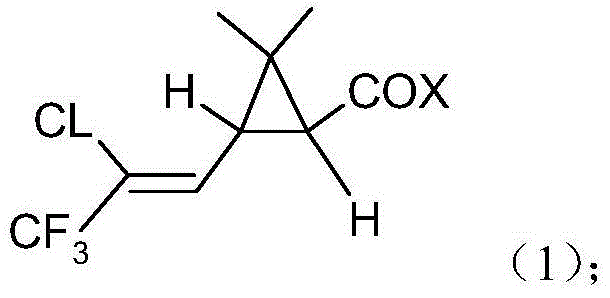
Provided is a novel preparation method of (Z)-7-chloro-((S)-2,2-dimethylcyclopropanecarboxamido)-2-heptenoic acid represented by the following formula (1), a key intermediate of cilastatin used as a supplement to imipenem. The novel preparation method of the invention produces a pure (Z)-7-chloro-((S)-2,2-dimethylcyclopropanecarboxamido)-2-heptenoic acid, a key intermediate of cilastatin, by selective hydrolysis of E isomers.
Owner:WISCHEM
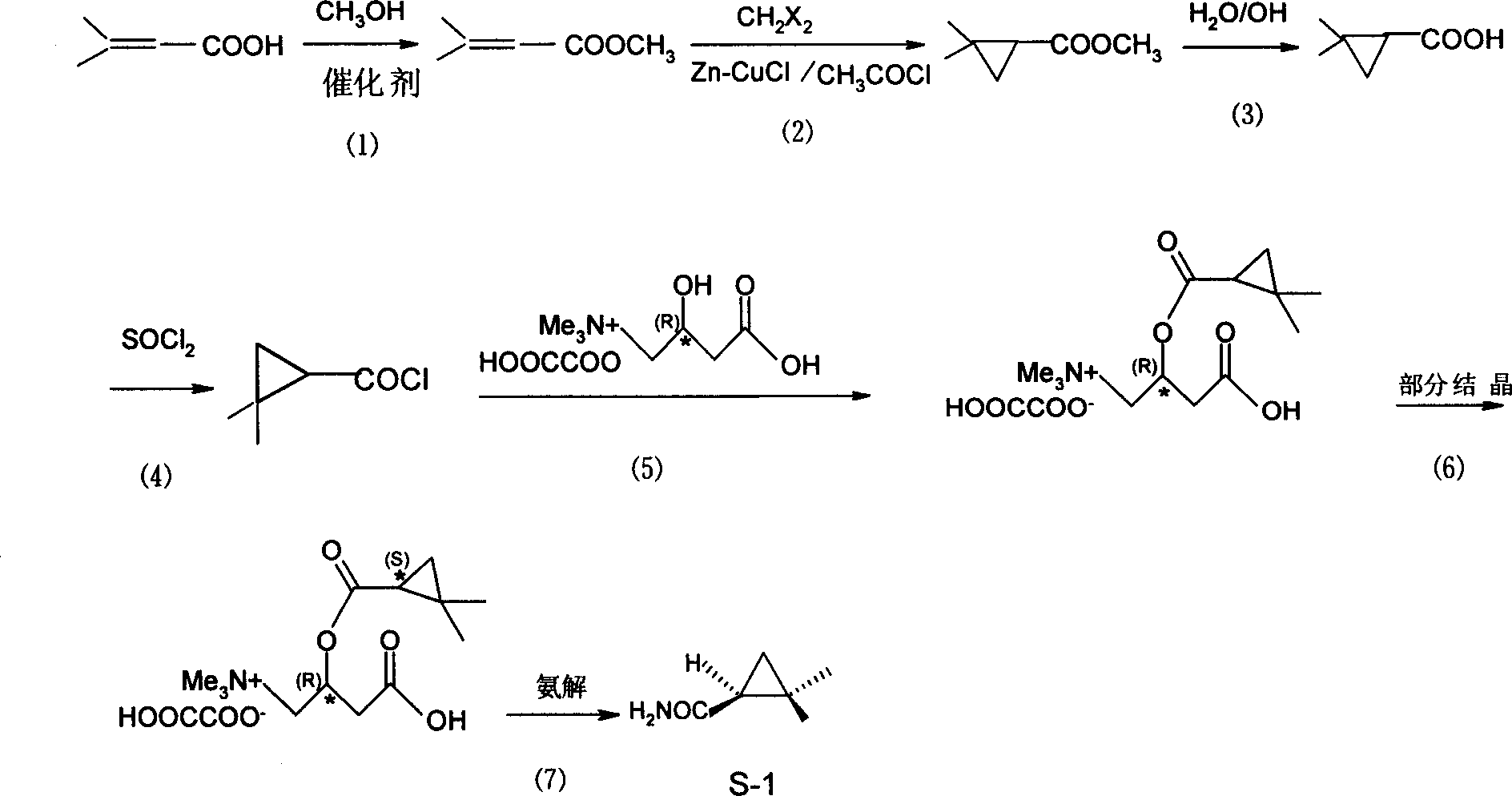
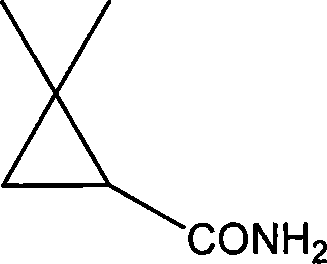
Method ofr synthesizing S-(1)-2.2 dimethylcyclopropane formamide
InactiveCN1562962AShort reaction timeMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationFormamideCyclopropanation
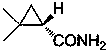
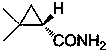
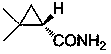
This invention relates to process for synthesizing S-(+)-2,2-dimethyl cyclopropane formyl amide, using penteneic acid as main raw material, after procedures of: esterification, cyclopropanization, hydrolyzation, acylation, salifying, partial crystallization and ammonolysis. advantages are: short reaction time, mild reaction condition, simple process, total yield is more than 15%, ee is larger than 95.6%.
Owner:ZHEJIANG UNIV +1
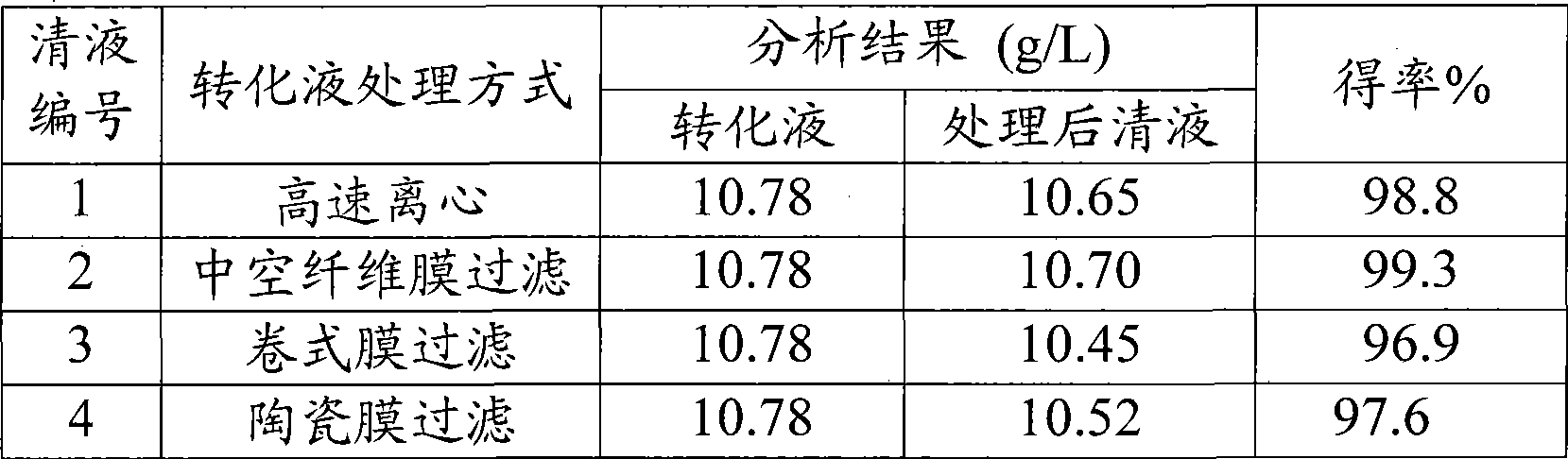
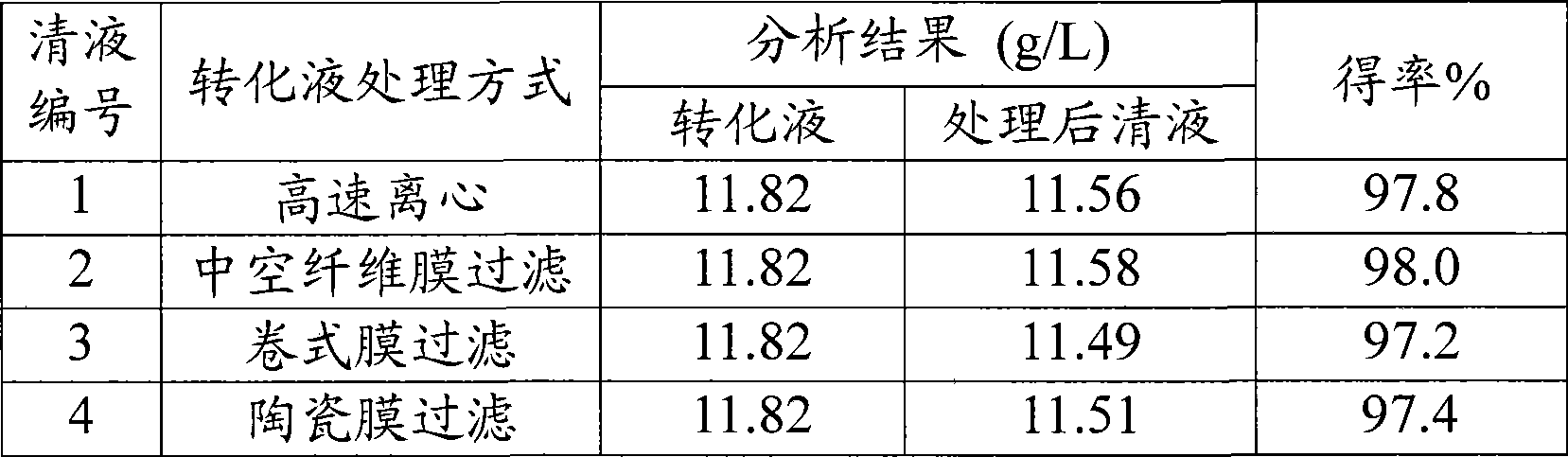
Method for purifying 2, 2-dimethyl-cyclopropane carboxamide produced by biocatalytic method
ActiveCN101445466ASimple production processHigh recovery rateMicroorganism based processesCarboxylic acid amide separation/purificationFiltrationSolvent
The invention relates to a method for purifying 2, 2-dimethyl-cyclopropane carboxamide produced by biocatalytic method. The method comprises: (1) conversion solution containing the 2, 2-dimethyl-cyclopropane carboxamide removes impurities by centrifugation or a membrane filtration system, so as to obtain supernatant fluid; (2) activated carbon which is 1-10 per mill of the quality of the supernatant fluid is added, and the decolorization of the activated carbon is carried out at 40-80 DEG C; (3) the pH value of the supernatant fluid after the decolorization is regulated to 8.5-13.5, macroporous adsorption resin is used for absorption till the supernatant fluid is saturated, the impurities are removed by water washing, hydrophilic solvent water solution with the volume concentration of 30-95 percent is further used for elution, the reduced pressure recovery of solvent and the concentration are carried out on eluent, so as to obtain a 2, 2-dimethyl-cyclopropane carboxamide crude product; (4) the 2, 2-dimethyl-cyclopropane carboxamide crude product is dissolved in hot water at 45-80 DEG C, stirred and crystallized at 0-4 DEG C, filtered and separated, and the 2, 2-dimethyl-cyclopropane carboxamide is finally obtained by drying crystals. The invention has the beneficial effects that the production process is simple and easy to operate, the product recovery rate is high and the industrial production is easy to realize.
Owner:ZHEJIANG UNIV OF TECH
Rhodococcus ZJPH1003 and application thereof in preparing S-(+)-2,2-dimethylcyclopropane carboxylic acid
ActiveCN102161978AEasy to trainReduce manufacturing costBacteriaMicroorganism based processesMicroorganismCarboxylic acid
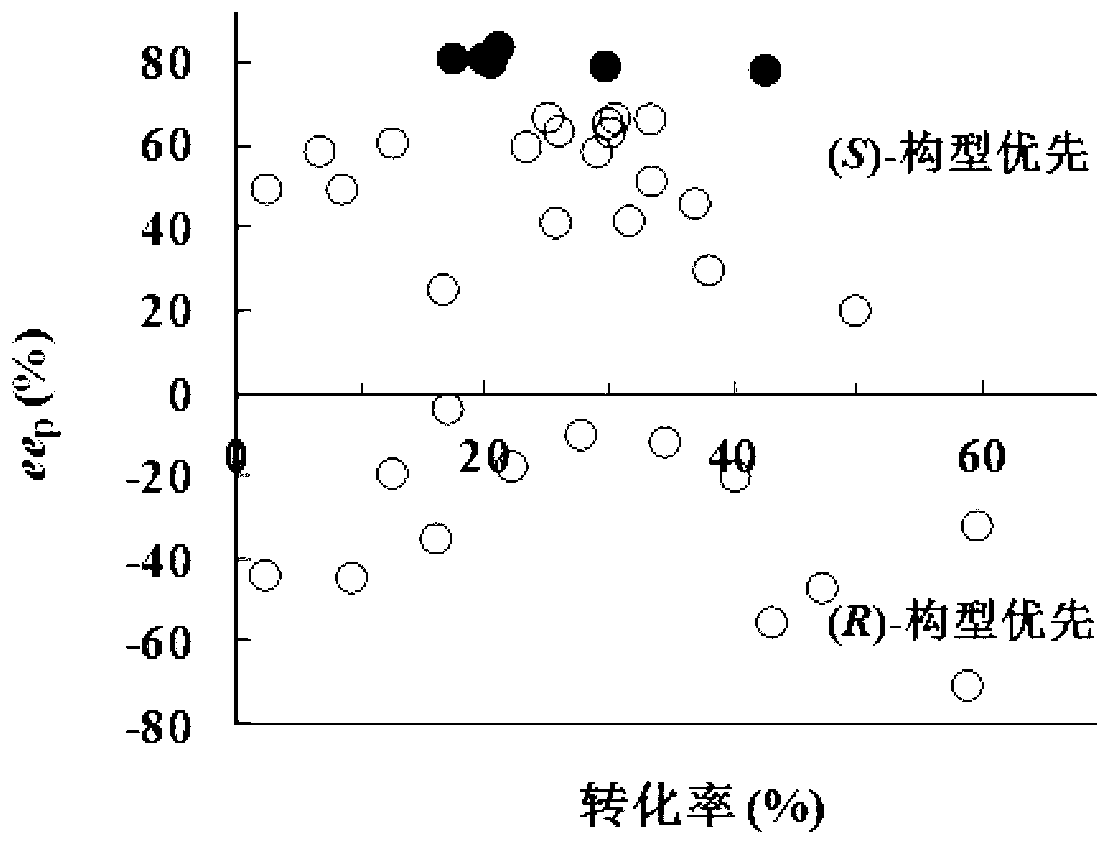
The invention provides a new strain-Rhodococcus sp. ZJPH1003 and an application thereof in preparing S-(+)-2,2-dimethylcyclopropane carboxylic acid through microbial catalytic asymmetric hydrolysis of 2,2-dimethylcyclopropane ethyl formate. The strain is conserved at China Center for Type Culture Collection, the conservation address is Wuhan University, Wuhan, China, postcode 430072, the conservation number is CCTCC M 2010371, and the conservation date is December 29, 2010. In the invention, the adopted method for preparing the S-(+)-2,2-dimethylcyclopropane carboxylic acid through microbial catalytic asymmetric hydrolysis of the 2,2-dimethylcyclopropane ethyl formate by utilizing the new microbial strain has the advantages of novel route, high yield, environmental friendliness and the like; and by utilizing a chiral biocatalysis method which takes a Rhodococcus ZJPH1003 cell as a catalyst, for the target product-S-(+)-2,2-dimethylcyclopropane carboxylic acid, the e.e can reach 82.5% and the yield can reach 41.3% when the substrate concentration is 30mmol / L.
Owner:ZHEJIANG UNIV OF TECH
Procymidone hapten and synthetic method and application thereof
InactiveCN109956895AImproving immunogenicityStrong specificityOrganic chemistryTesting foodHydrolysisVaccine Immunogenicity
The invention discloses a procymidone hapten and a synthetic method and application thereof. The synthetic method comprises the following steps: (1) alpha-chloromethyl acrylate and methyl methacrylateare used as starting materials to synthesize 1,2-dimethyl-1,2-cyclopropane dicarboxylic methyl ester; 1,2-dimethyl-1,2-cyclopropane dicarboxylic methyl ester undergoes hydrolysis and intramolecular condensation to generate 1,2-dimethylcyclopropane-1,2-dicarboxylic acid anhydride; (2) 3-chloro-5-nitrophenol and a compound 3 are used as raw materials to synthesize an intermediate with an arm, and the intermediate is subjected to a reduction reaction to obtain an intermediate B; and (3) 1,2-dimethylcyclopropane-1,2-dicarboxylic acid anhydride and the intermediate B undergo two times of condensation reaction for cyclization and one time of hydrolysis reaction to obtain the final target product. In the invention, characteristic structure of procymidone is retained in the synthesized procymidone hapten such that the immunogenicity of the procymidone hapten is obviously enhanced, and the procymidone hapten also has a carboxyl group capable of coupling with a carrier, thus providing a foundation for subsequently building various immunoassay methods of procymidone.
Owner:SHENZHEN BIOEASY BIOTECHNOLOGY CO LTD
Spray for controlling sanitary insect pests
ActiveCN102578133ATimely supplementSustainably play the role of repelling pestsBiocidePest repellentsMethylcyclopropaneCarboxylic ester
The invention discloses a spray for controlling sanitary insect pests. The spray comprises an active ingredient and a dispensing medium, wherein the active ingredient is one or a combination of both of 2,3,5,6-tetrafluoro phenmethyl(1R,3S)-3-(2,2-dichloro ethylene)-2,2-dimethyl cyclopropane carboxylic ester and 2,3,5,6-tetrafluoro-4-methoxymethyl phenmethyl-3-(3,3,3-trifluoro-1-allyl)-2,2-dimethyl cyclopropane-carboxylic acid ester. An insect pest prevention and control active compound with more superior physicochemical properties is directly dissolved or emulsified in the dispersing medium, and is prepared into a spray, so that the medicinal effect and environmental friendliness of the spray are improved. The invention further discloses a sanitary insect pest control spray which has an aging indicating effect. An indicator is added into the spray, so that the effect lasting time of a liquid medicament can be indicated intuitively.
Owner:中山榄菊日化实业有限公司
Method for synthesizing cilastatin sodium
The invention discloses a method for synthesizing cilastatin sodium, comprising the following steps: preparing (Z)-7-iodine-2-((S)-2, 2-dimethyl cyclopropane formamido)-2-heptenoic acid alkyl ester by reaction of (Z)-7-chlorine-2-((S)-2, 2-dimethyl cyclopropane formamido)-2-heptenoic acid alkyl ester and sodium iodide; enabling (Z)-7-iodine-2-((S)-2, 2-dimethyl cyclopropane formamido)-2-heptenoic acid alkyl ester, cysteine alkyl ester hydrochloride, alkali and a solvent to react to obtain cilastatin dialkyl ester; and enabling the cilastatin dialkyl ester and sodium hydroxide to react to obtain cilastatin sodium. The alkali used in the method can be one of or more of K2HPO4, CsCO3 and K3PO4, thereby avoiding the usage of strong alkali and an anhydrous reaction system and realizing mild reaction and easy operation. The method has the advantages of good economical efficiency, mild reaction conditions, high yield, little three wastes (waste gas, waste water and industrial residue) and no pollution and can be used for industrialization production. The product can be separated easily and has high purity.
Owner:ZHEJIANG NORMAL UNIVERSITY
Bi-cyclopropyl compound as well as preparation method and application thereof
ActiveCN110041158AImprove performanceGood thermal stabilityHydrocarbon by hydrogenationCatalystsMethylcyclopropaneEthyl phosphate
The invention discloses bi-cyclopropyl compound. The bi-cyclopropyl compound is of a following structure; the bi-cyclopropyl compound is named as 2-(3-cyclopropyl butyl)-1,1-dimethyl cyclopropane and / or named as 2-(2-(1-ethyl cyclopropyl)ethyl)-1,1-dimethyl cyclopropane and / or named as 1-(4-methyl pentyl)-1,1-bi-cyclopropane. The invention further discloses a preparation method and application ofthe bi-cyclopropyl compound.
Owner:TIANJIN UNIV
Preparation method of (S)-(+)-2,2-dimethylcyclopropanecarboxylic acid by biocatalysis
The invention discloses a novel preparation method of (S)-(+)-2,2-dimethylcyclopropanecarboxylic acid by biocatalysis. The method uses ethyl 2,2-dimethylcyclopropanecarboxylate as substrate and Novozym 435 lipase as biological catalyst, then biological catalyst asymmetric hydrolysis reaction is carried out on the mixture at a temperature of 25-45 DEG C and at pH ranging from 6.0-8.0 in a water phase reaction system, after the reaction is completed, the reaction solution is isolated to obtain (S)-(+)-2,2-dimethylcyclopropanecarboxylic acid. The asymmetric hydrolysis reaction utilizes Novozym 435 lipase to catalyze ethyl 2,2-dimethylcyclopropanecarboxylate, therefore, the enzyme specificity is strong, the catalytic efficiency is high, no side product is generated, and the optical purity and yield of the product are both higher than those of the product obtained by chemical methods.
Owner:ZHEJIANG UNIV OF TECH
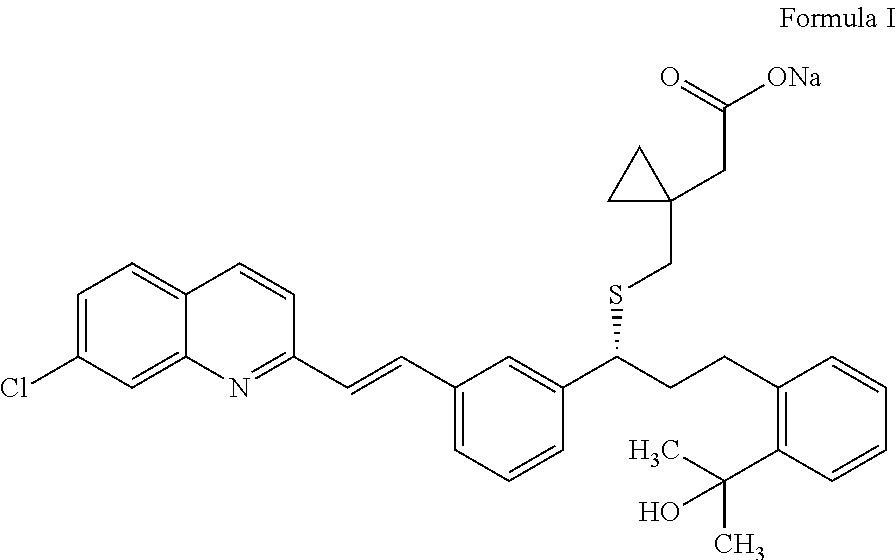
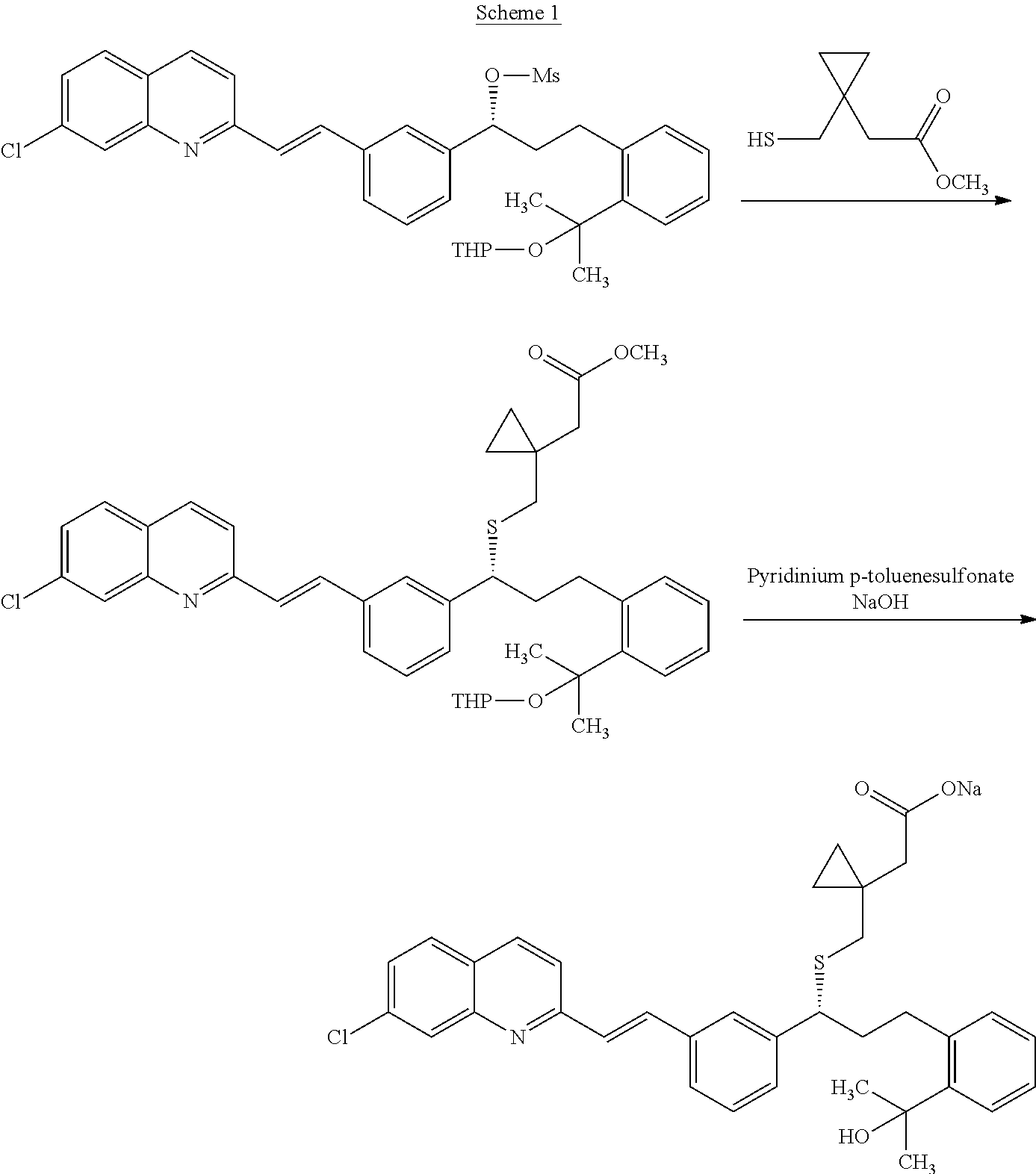
Process for preparation of montelukast sodium
ActiveUS20150299127A1High purityHigh yieldOrganic chemistryRespiratory disorderSulfonyl chloridePropanol
Disclosed is a process for the preparation of montelukast sodium. The process comprises a) reacting 2-(2-(3(S)-(3-(2-(7-chloro-2-quinolinyl)-ethenyl)phenyl)-3-hydroxypropyl)phenyl)-2-propanol with methane sulfonyl chloride and coupling the resultant mesylate compound with 1-(mercaptomethyl)cyclopropane acetic acid in presence of a base and free alkali source followed by saltification with an amine in a single step reaction and b) converting the montelukast amine salt to montelukast sodium salt.
Owner:LAURUS LABS
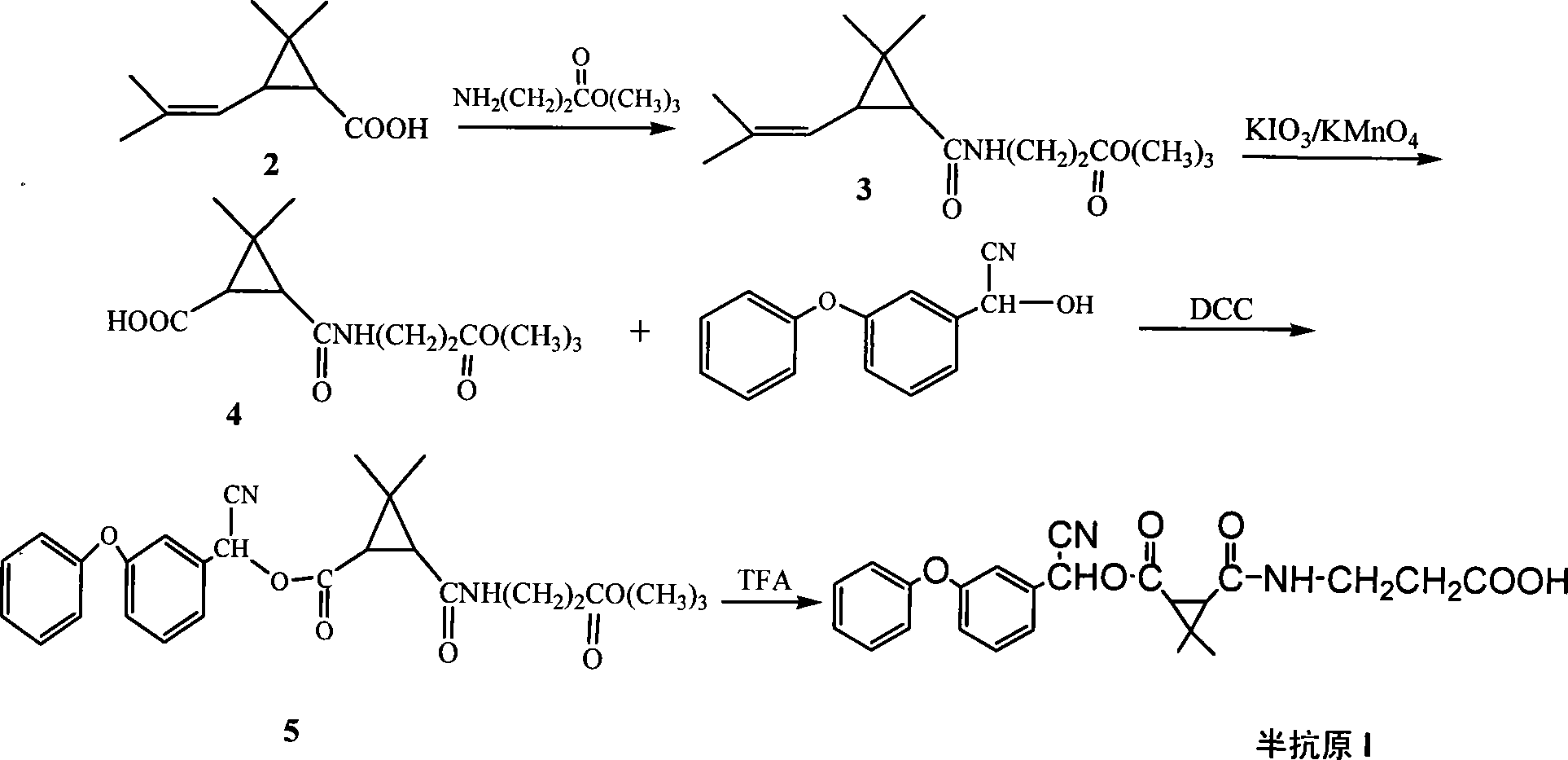
Cell strain 16, monoclonal antibody produced by cell strain 16 and use of monoclonal antibody produced by cell strain 16
ActiveCN103911348AHigh sensitivityStrong specificityMicroorganism based processesTissue cultureBovine serum albuminAmmonium sulfate precipitation
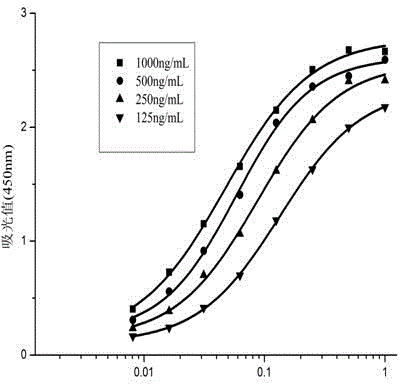
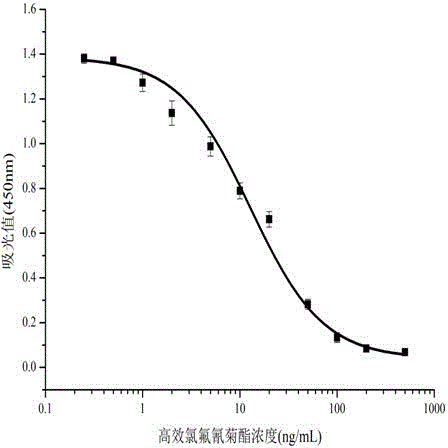
The invention relates to a cell strain 16, a monoclonal antibody produced by the cell strain 16 and a use of the monoclonal antibody produced by the cell strain 16. A preparation method of the cell strain 16 comprises the following steps of coupling hapten 1 (H1) 3-[cyano[cis-3-[2-chloro-3,3,3-trifluoroethyl-2, dimethyl]cyclopropane-phenoxy]phenylpropionic acid and hemocyanin KLH by an active ester method, coupling hapten 2 (H2) cyano-[3-(4-aminophenoxy)phenyl-cis-3-[2-chloro-3,3,3-trifluorovinyl-2,2-dimethyl]cyclopropionate and bovine serum albumin by a diazotization method to obtain a conjugate as a coating antigen, and carrying out animal immunization, serum determination, cell fusion, screening and subcloning to obtain the cell strain 16. Through a mouse internal ascites induced-production method, an ascitic fluid is produced, and then through a caprylic acid-ammonium sulfate precipitation method, the ascitic fluid is purified so that the corresponding monoclonal antibody is produced. The monoclonal antibody has high sensitivity, median inhibitory concentration (IC50) of 13.26+ / -1.23ng / mL and strong singularity, provides a technology for detection of high-efficiency cyhalothrin residue in domestic agricultural products and has a good market prospect.
Owner:HENAN UNIV OF SCI & TECH
Synthesizing method of S-(+)-2, 2-dimethylcyclopropane carboxamide
ActiveCN101735099AShort reaction timeMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationGlycine ethyl esterCyclopropanation
The invention relates to a synthesizing method of S-(+)-2, 2-dimethylcyclopropane carboxamide which is synthezied by using glycine ethyl ester hydrochloride as main starting material and sequentially comprising the following steps of: diazotization reaction, cyclopropanation reaction, hydrolysis reaction, acylation reaction, resolution reaction and ammonolysis reaction. The synthesizing method has short reaction time, moderate reaction condition, simple process, overall yield more than 17 percent and ee more than 98 percent.
Owner:JIANGSU YUXIANG CHEM
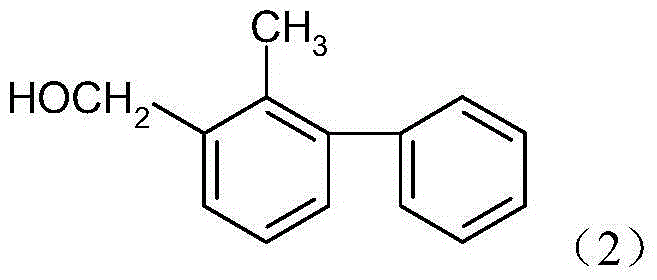
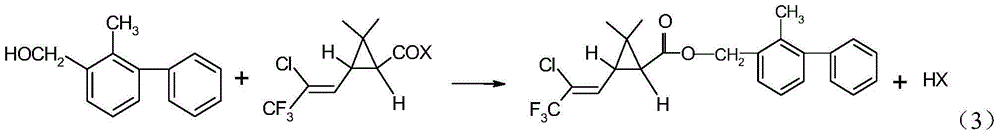
Method for producing bifenthrin with clean synthesizing process
InactiveCN104628569AHigh synthetic conversion rateHigh product contentPreparation from carboxylic acid halidesHalogenSolvent
The invention provides a method for producing bifenthrin with a clean synthesizing process. According to the method, 3-(2-chloro-3,3,3-trifluoro-1-allyl)-2,2-dimethylcyclopropane formyl halide and 2-methyl-3-biphenylmethanol are adopted to directly react in one solvent or mixed solvent of more than two solvents in favor of overflow of halogen hydride, halogen hydride generated in the reaction is removed from tail gas in time by means of negative pressure, and the product is directly cooled and crystallized to obtain bifenthrin. The reaction equation is as shown in a formula (3), wherein X is Cl or Br. The production method has the characteristics of high product content, simple process flow, small output of the 'Three Wastes' and the like, and accords with the requirement for clean production.
Owner:JIANGSU YANGNONG CHEM +1
Epidermis brevibacterium ZJB-07021 and use for preparation of (S)-2,2-dimethyl cyclopropane formamide thereof
The invention provides a novel strain which is Brevibacterium epidermidis ZJB-07021 and the application in the microbiological preparation of (S)-2, 2-dimethylcycloproprane carboxamide. The beneficial effects of the invention mainly lie in that the invention provides a novel strain which has stereo selectivity in the production of optically pure (S)-2, 2-dimethylcycloproprane carboxamide; and the strain can be used for the production of optically pure (S)-2, 2-dimethylcycloproprane carboxamide. The invention obtains a novel strain which is different from the previous research through screening; the invention provides the basis for the investigation on the variety of microbial strain in the chiral resolution aspect and the further comparison of the difference of transformation pathway and reaction mechanism among various strains.
Owner:ZHEJIANG UNIV OF TECH +1
Composition for treating respiratory and skin diseases, comprising at least one leukotriene antagonist and at least one antihistamine
A pharmaceutical composition useful in the treatment of sneezing, itching runny nose, nasal congestion, redness of the eye, tearing, itching of the ears or palate, shortness of breath, inflammation of the bronchial mucosa, reduced Forced Expiratory Volume In One Second (FEV1), coughs, rash, itchy skin, headaches, and aches and pains associated with seasonal allergic rhinitis, perennial allergic rhinitis, common colds, otitis, sinusitus, allergy, asthma, allergic asthma and / or inflammation, in a mammalian organism in need of such treatment. The composition comprises: i) an effective amount of at least one leukotriene antagonist selected from a) montelukast, b) 1-(((R)- (3-(2-(6,7- difluoro-2- quinolinyl)ethenyl) phenyl)-3-(2- (2-hydroxy-2- propyl)phenyl) thio)methylcyclopropaneacetic acid; c) 1-(((1(R)-3 (3-(2-(2,3- dichlorothieno[3, 2-b]pyridin-5-yl) -(E)-ethenyl)phenyl) -3-(2-(1-hydroxy-1- methylethyl) phenyl)propyl) thio)methyl) cyclopropaneacetic acid; d) pranlukast; or f) [2-[[2-(4-tert -butyl-2-thiazolyl) -5-benzofuranyl] oxymethyl]phenyl] acetic acid; or a pharmaceutically acceptable salt thereof; in admixture with ii) an effective amount of at least one antihistamine which is descarboethoxyloratidine, cetirizine, fexofenadine, ebastine, astemizole, norastemizole, epinastine, efletirizine or a pharmaceutically acceptable salt thereof.
Owner:SCHERING AG
Method for synthesizing pyrethroid hapten compounds
InactiveCN101215247AQuick checkCarboxylic acid nitrile preparationOrganic compound preparationGamma-Aminobutyric acidAcetonitrile
The invention discloses a synthesis process of pyrethroids hapten compound by using dimethyl trichloromethyl chloroformate, gamma-aminobutyric acid and as main material, which comprises following steps that firstly generating 3-(2, 2-dimethyl-3-(2'-methacryloyl) cyclopropyl carbonyl amino) ethyl propionate, secondly generating 3-(3-ethyoxyl-3-oxopropanecarbonic acyl radical)-2, 2-dimethyl cyclopropane aminic acid, thirdly generating 2-hydroxy-2-(3-pheonexyphenyl) acetonitrile, fourthly generating cyano-(3-phenoxy phenyl) methyl N-2-ethyoxylcarbonylethyl-2, 2-dimethylcypromethyl carbonate, fifthly generating 3-(3-(( cyano(3-phenoxyphenyl) methoxyl) carbonic acyl radical)-2, 2-phenoxy phenylcypro carbamoyl) ethylformic acid. Hapten which is prepared by the invention comprises three similarity structures of most pyrethroids pesticides, ester with-CN and three-membered ring structure.
Owner:ZHEJIANG UNIV
Aryloxmethyl cyclopropane derivatives as PDE10 inhibitors
The present invention is directed to aryloxymethyl cyclopropane derivatives which are useful as therapeutic agents for the treatment of central nervous system disorders associated with phosphodiesterase 10 (PDE10). The present invention also relates to the use of such compounds for treating neurological and psychiatric disorders, such as schizophrenia, psychosis or Huntington's disease, and those associated with striatal hypofunction or basal ganglia dysfunction.
Owner:MERCK SHARP & DOHME LLC
Preparation method of chiral dimethyl cyclopropyl carboxamide
InactiveCN104193645AOrganic compound preparationCarboxylic acid amide separation/purificationAlkyl transferDiazoacetic ester
The invention discloses a preparation method of chiral dimethyl cyclopropyl carboxamide. The method comprises a step of asymmetric cyclopropyl alkylation and a step of catalytic amidation of cyclopropyl formic ether, wherein in the step of asymmetric cyclopropyl alkylation, a cyclopropyl alkylation reaction is carried out on ethyl diazoacetate and isobutene under the catalysis of a chiral ligand complex of a cuprous salt so as to obtain (S)-dimethyl cyclopropyl formate; and in the step of catalytic amidation of cyclopropyl formic ether, an ammonolysis reaction is carried out on the (S)-dimethyl cyclopropyl formate by one step so as to directly obtain (S)-2,2-dimethyl cyclopropyl carboxamide, and refining the carboxamide with an alcohol so as to obtain the chiral dimethyl cyclopropyl carboxamide with chemical purity being greater than 99.5% and an e.e. value being greater than 99.5%. Thus, the method used for synthesizing the (S)-2,2-dimethyl cyclopropyl carboxamide is environment-friendly, simple, rapid and efficient.
Owner:SHANGHAI INST OF TECH
New pyrethroid compound and preparation method and application
ActiveCN101665433AHigh activityEasy to solveBiocideOrganic compound preparationAbsolute configurationChemical compound
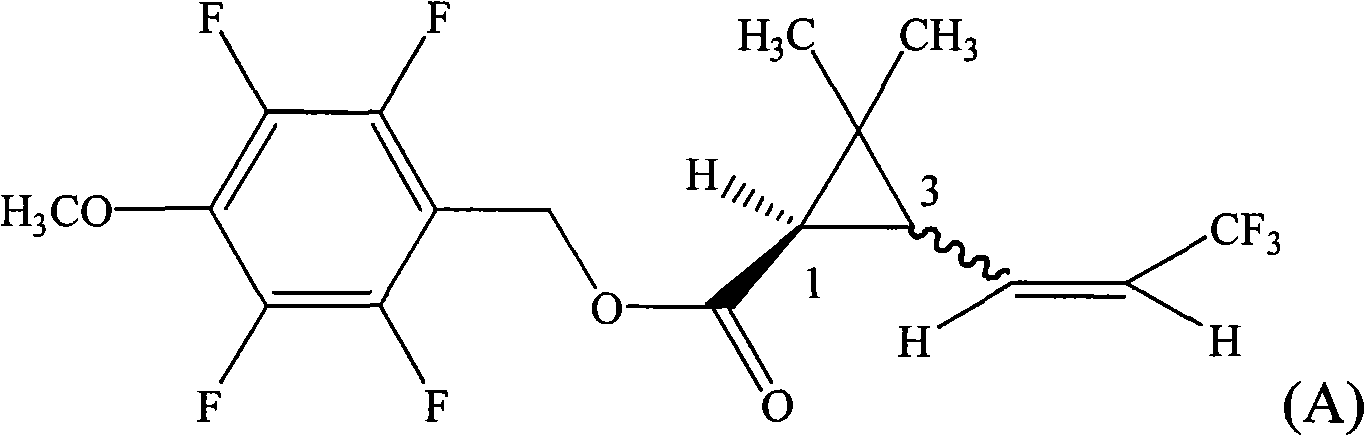
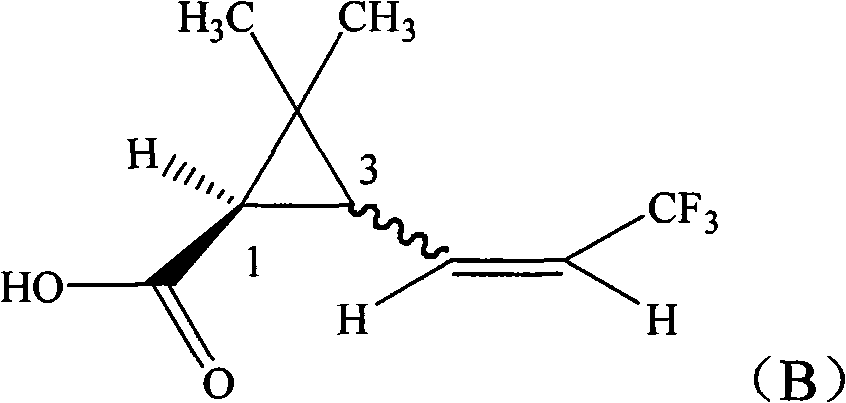
The invention provides a pyrethroid compound which is a stereoisomer of 2,3,5,6-tetrafluoro-4-methoxybenzyl-3-(3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropane carboxylate. The compound is characterized in that the structure of the compound is shown in formula (A), wherein the carbon-carbon double bond in the carboxylic acid part of the formula (A) is Z configuration, the absolute configuration of the 1-site of cyclopropane is R configuration; the compound is 2,3,5,6-tetrafluoro-4-methoxybenzyl-1R-(Z)-3-(3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropane carboxylate. The pyrethroid compound has high activity and significant effect for controlling the pestiferous pests. The invention also provides a preparation method and an application of the pyrethroid compound.
Owner:JIANGSU YANGNONG CHEM +1
Rhodococcus and application thereof in preparation of (S)-(+)-2,2-dimethylcyclopropane carboxylic acid
InactiveCN102757924AGood catalyticImprove stabilityBacteriaHydrolasesCarboxylic acidBacterial strain
The invention discloses a rhodococcus (Rhodococcus sp.) bacterial strain ECU 1013, and the preservation serial number of the bacterial strain is CGMCC No.5911. The invention also discloses a preparation method of esterase of the rhodococcus and a method for catalyzing and preparing the (S)-(+)-2,2-dimethylcyclopropane carboxylic acid [(S)-(+)-DMPCA for short]. The rhodococcus bacterial strain ECU 1013 and the methods have the advantages that 2,2- dimethylcyclopropane carboxylic acid ester is catalyzed by using the rhodococcus and the esterase of the rhodococcus, enantioselective hydrolysis is performed and the (S)-(+)-DMPCA is prepared, the process has the advantages of being high in specifity, good in the catalyzing effect, mild in reaction condition, and the like. The optical purity of the target product (S)-(+)-DMPCA is 98% ee, and the single splitting is above 40%.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com