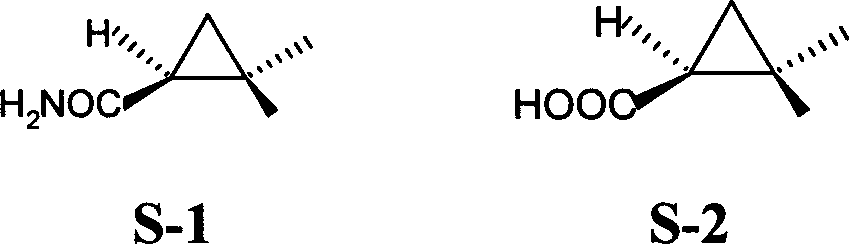Method ofr synthesizing S-(1)-2.2 dimethylcyclopropane formamide
A synthesis method and cyclopropanation technology, which is applied in the preparation of carboxylic acid amides, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of cumbersome operation, difficult preparation, and high price, and achieve mild reaction conditions and simplified disassembly. Step-by-step, short reaction time results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
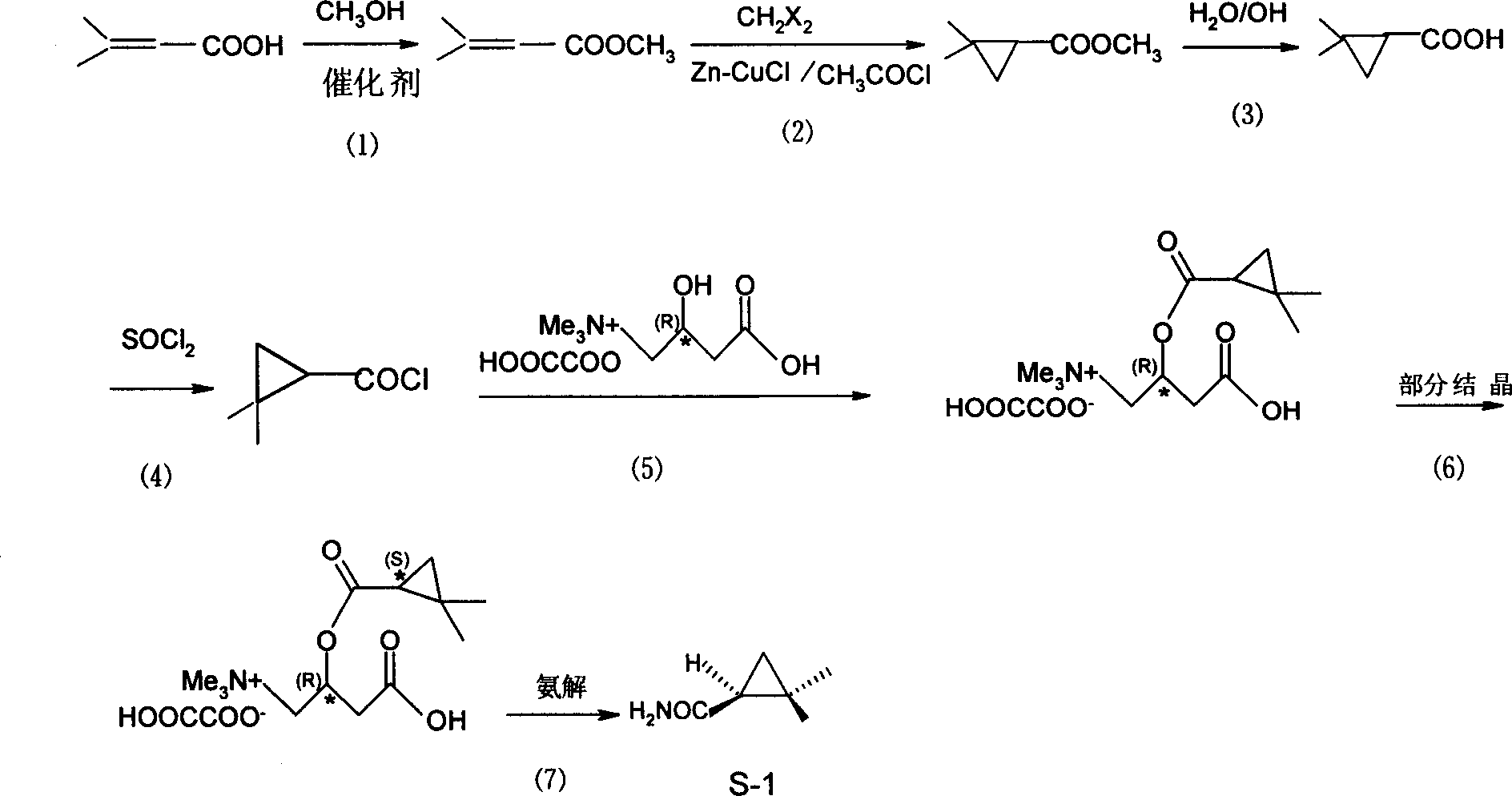
[0025] Embodiment 1: a kind of S-(+)-2, the synthetic method of 2-dimethylcyclopropanecarboxamide is characterized in that: take prenylic acid as main starting raw material, make through following steps successively:
[0026] (1) Esterification, i.e. the preparation of methyl methacrylate:
[0027] Isopentenoic acid (10.0 g, 0.1 mol) was dissolved in methanol (9.6 g, 0.3 mol), and phosphorus oxychloride (4.0 mL) was added dropwise at a reflux temperature of 60°C to 80°C, and the dropwise was completed in 30 minutes. It takes 4 to 5 hours to maintain the reflux temperature until no hydrogen chloride gas escapes. After the reaction was completed, the reaction solution was salted out by adding a saturated sodium chloride solution, and the upper organic layer was separated, neutralized with a saturated sodium carbonate solution, and then washed with a saturated sodium chloride solution until neutral. 10.5 g of crude product was obtained, with a content of about 85%. Rectify unde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com