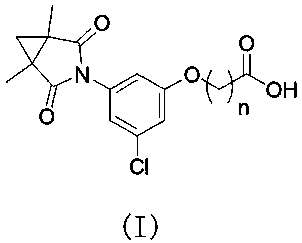
Procymidone hapten and synthetic method and application thereof
A synthesis method, the technology of procymidone, is applied in the direction of material inspection products, test food, instruments, etc., to achieve the effects of simple reaction operation, synthetic cost advantages, and easy control of reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
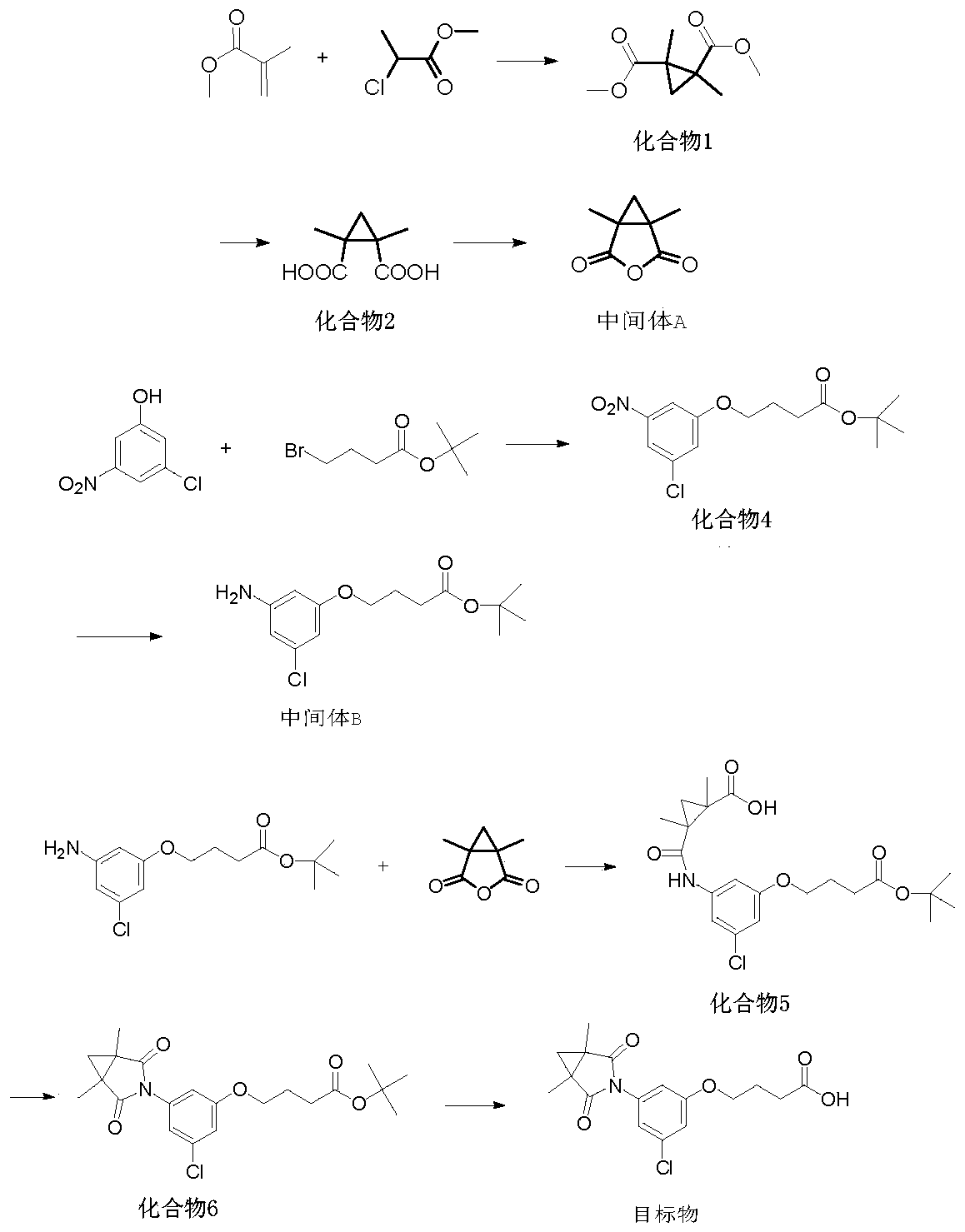
[0041] In the second aspect, the present invention also provides a method for synthesizing the above-mentioned procymidone hapten, comprising the following steps:
[0042] S1, providing an intermediate A having the structural formula ;
[0043] S2, providing an intermediate B having the structural formula , wherein, n is the number of -CH2 groups, and n is an integer of 1-5;
[0044] S3, the intermediate A and intermediate B are reacted to obtain compound 5, and the compound 5 has the structural formula , wherein, n is the number of -CH2 groups, and n is an integer of 1-5;
[0045] S4, the compound 5 is subjected to a condensation reaction to obtain a compound 6, and the compound 6 has the structural formula , wherein, n is the number of -CH2 groups, and n is an integer of 1-5;
[0046] S5, subjecting the compound 6 to a hydrolysis reaction to obtain the procymidone hapten.
[0047] According to the structural characteristics of procymidone, the present invention ratio...
Embodiment 1
[0067] A synthetic method of procymidone hapten, comprising the steps of:
[0068] S1, providing intermediate A, which is 1,2-dimethyl-1,2-cyclopropane anhydride;
[0069] The preparation method of the intermediate A is:
[0070] (1) Add 100mL toluene to a 250mL three-necked flask, add 0.2mol sodium hydride in an ice-water bath, stir for 30 minutes, then slowly add 0.1mol α-methyl chloroacrylate and 0.12mol methyl methacrylate dropwise, and the addition is complete After the reaction at room temperature overnight, after the reaction is complete, add 10mL of ethanol, stir for 10-15min, then add 200mL of water, adjust the pH of the solution to 5-6 with dilute hydrochloric acid, extract with 200mL ethyl acetate, collect the ethyl acetate layer, acetic acid The ethyl ester layer was washed with saturated brine, dried over anhydrous sodium sulfate, and then distilled under reduced pressure to remove toluene and ethyl acetate, and the residue was purified by distillation under redu...
Embodiment 2
[0080] A synthetic method of procymidone hapten, comprising the steps of:
[0081] S1, providing intermediate A, which is 1,2-dimethyl-1,2-cyclopropane anhydride;
[0082] The preparation method of the intermediate A is:
[0083] (1) Add 100mL of dichloromethane into a 250mL three-necked flask, add 0.5mol of sodium hydride in an ice-water bath, stir for 30min, then slowly add 0.1mol of α-methyl chloroacrylate and 0.1mol of methyl methacrylate dropwise, drop After the addition, react overnight at room temperature. After the reaction, add 20 mL of ethanol, stir for 10-15 minutes, add 200 mL of water, adjust the pH of the solution to 5-6 with dilute hydrochloric acid, extract with 200 mL of ethyl acetate, and collect the ethyl acetate layer , the ethyl acetate layer was washed with saturated brine, dried over anhydrous sodium sulfate, dichloromethane and ethyl acetate were removed by vacuum distillation, and the residue was purified by oil pump vacuum distillation to obtain 1,2-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com