Preparation method of cilastatin sodium
A technology of cilastatin sodium and sodium hydroxide, applied in the field of preparing cilastatin sodium, can solve the problems of reducing the yield and purity of the product, being lengthy, and the operation of the purification method is cumbersome, so as to improve the chromaticity and purity, avoid the cumulative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
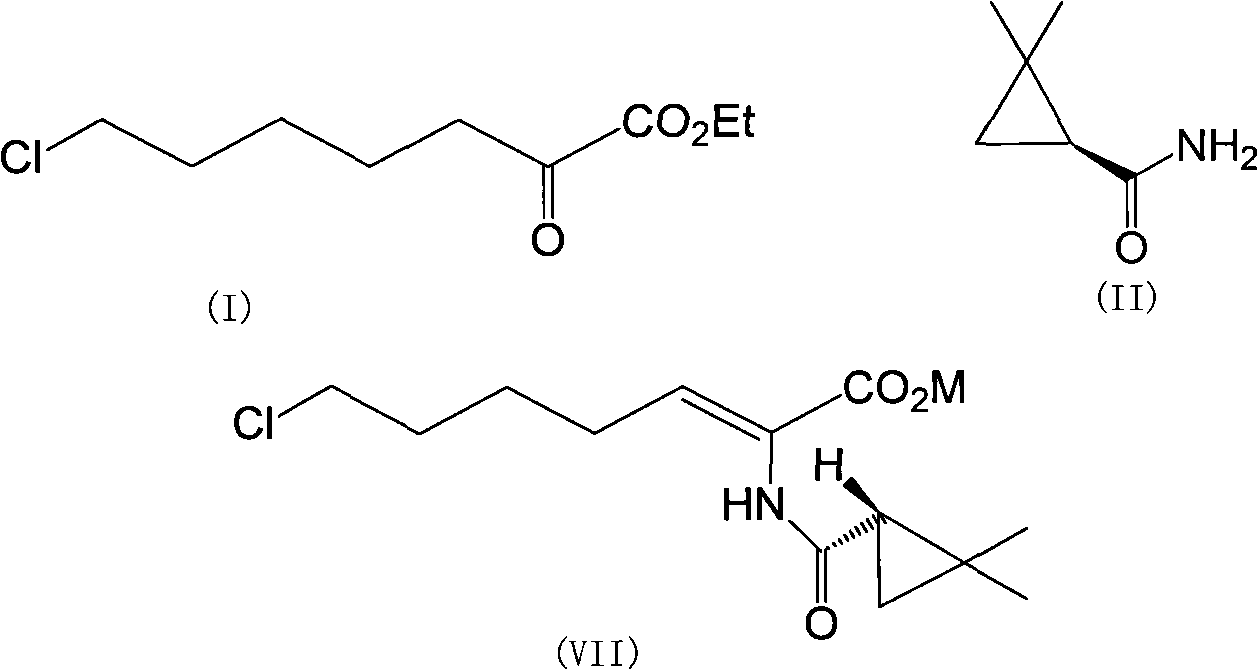
[0027] The preparation of (Z)-7-halo-2((S)-2,2-dimethylcyclopropanecarboxamido)-2-heptenoic acid sodium crystals comprises the following steps:
[0028] 1. 100g 7-chloro-2-oxoheptanoic acid ethyl ester, 53g (S)-2,2-dimethylcyclopropanecarboxamide and 1.3g p-toluenesulfonic acid were refluxed in 600ml toluene at 130°C for 10 Hour. After the reaction was completed, the toluene was concentrated and recovered. Add 300ml of sodium hydroxide solution (10%) to the concentrate, monitor the reaction process by HPLC, and react at 30-45°C for 8 hours. 100 ml of toluene was added to the reaction solution obtained by hydrolysis each time, and the reaction solution was washed repeatedly three times. The organic phase was discarded, acidified by adding concentrated hydrochloric acid, and the pH of the aqueous phase was adjusted to 3.5. The liquid layer was extracted three times with 280ml of toluene, the aqueous phase was discarded, and the organic phase was dried over anhydrous sodium su...
Embodiment 2
[0031] After obtaining the hydrolysis reaction liquid by the method described in Step 1 of Example 1, add 150 ml of dichloromethane each time, wash the hydrolyzate repeatedly 3 times, discard the organic phase, and add concentrated sulfuric acid to adjust the pH of the aqueous phase to 3.5. The material-liquid layer was extracted three times with 800 ml of ethyl acetate, and the aqueous phase was discarded. The organic phase was dried over anhydrous sodium sulfate, filtered and distilled under reduced pressure. Add 500ml of acetonitrile to the concentrate, fully stir to dissolve, and filter to remove insoluble impurities. Slowly add 30% potassium hydroxide solution to the above filtrate until the pH of the solution is 7.0, stop adding the potassium hydroxide solution dropwise, continue stirring for 1.0 hour, and concentrate the reaction solution under reduced pressure after the pH is stable. The concentrated solution was crystallized at low temperature, and a large amount of ...
Embodiment 3
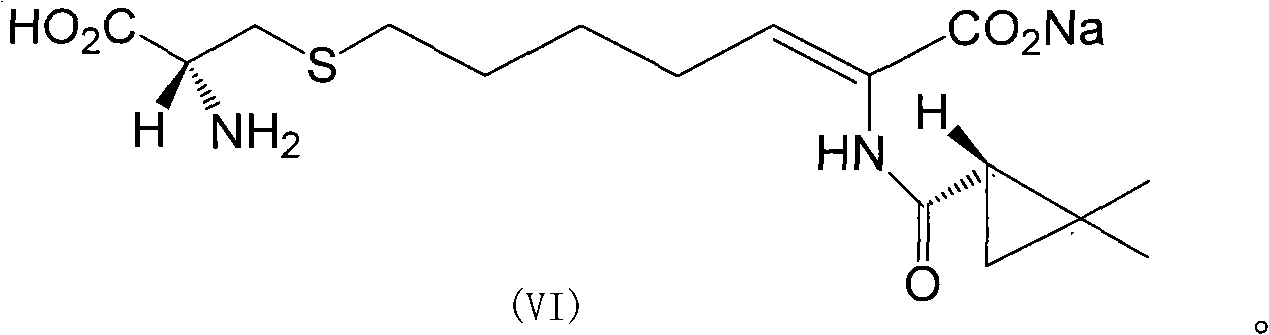
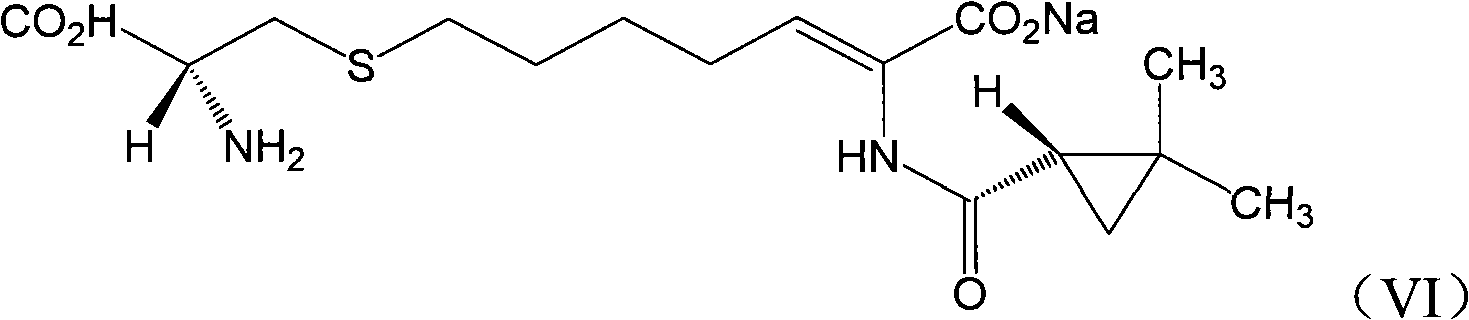
[0033] 1.50g (Z)-7-chloro-2((S)-2,2-dimethylcyclopropanecarboxamido)-2-heptenoic acid sodium solid was dissolved in 350ml10% sodium hydroxide solution, until all After dissolving, 28 g of L-cysteine hydrochloride was added, and the reaction was incubated at 55° C. for 8 hours. The condensation reaction solution was cooled to room temperature, acidified with 3N hydrochloric acid, and the pH of the solution was adjusted to 3.0. Add 150ml of petroleum ether to wash the acidified solution, discard the organic layer, and concentrate the solution volume under reduced pressure to 1 / 2 of the original volume. The obtained solution was loaded onto an AP400 macroporous resin adsorption column, and washed with distilled water until the conductivity of the effluent was less than 10 μs / cm.
[0034] 2. Slowly elute the above-mentioned macroporous adsorption resin with 0.3N sodium hydroxide solution (440ml), and collect the obtained cilastatin sodium solution. Add 1.3 g of activated carbo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com