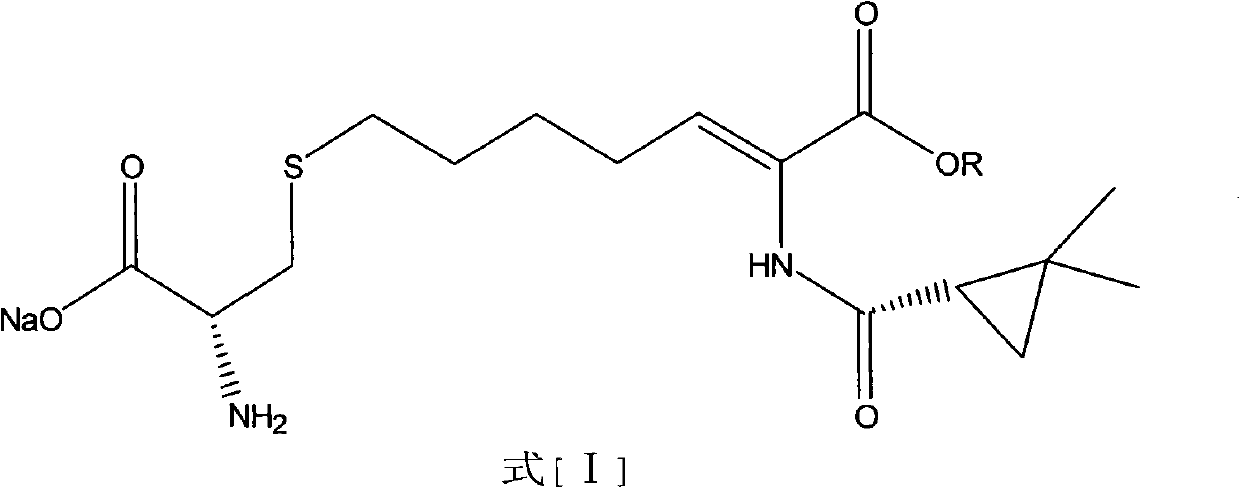
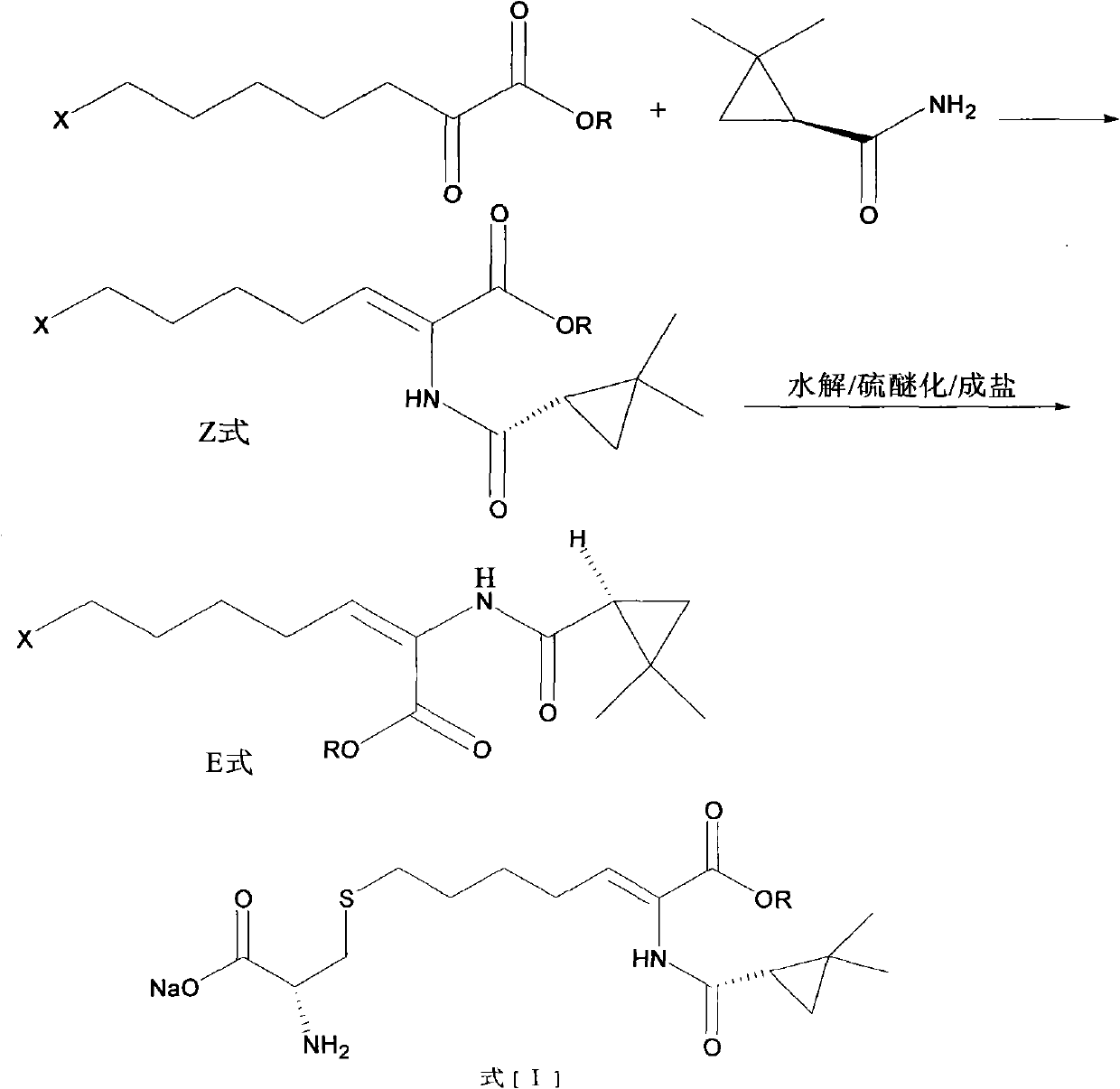
Preparation method of cilastatin sodium
A technology of cilastatin sodium and dimethylcyclopropanecarboxamide, applied in the field of pharmaceutical synthesis, can solve the problems of low conversion rate, difficult to remove impurities, easy generation of impurities and the like, achieves improved yield and purity, and reduced solvent content , the effect of simplifying the purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1 cilastatin sodium
[0025] Step (1) 25g ethyl 7-chlorooxyheptanoate, 13.68g(s)-2,2-dimethylcyclopropanecarboxamide and 0.14g p-toluenesulfonic acid were dissolved in toluene solution, and the temperature was refluxed at 110°C After the condensation was completed, the reaction mixture was washed with dilute hydrochloric acid and a saturated aqueous solution of sodium bisulfite, and then concentrated to recover toluene. The viscous was hydrolyzed in 20ml of ethanol and 25ml of 50% sodium hydroxide aqueous solution for 12h, then adjusted to pH 3.0-4.0 with concentrated hydrochloric acid, extracted three times with 30ml of ethyl acetate, and concentrated to obtain 31.2g of viscous.
[0026] Step (2) Add 20 g of cysteine hydrochloride and 120 ml of 2N sodium hydroxide solution to the viscous material in the previous step, and stir and react at room temperature for 12 hours under nitrogen protection;
[0027] Step (3) Use hydrochloric acid t...
Embodiment 2
[0029] The preparation of embodiment 2 cilastatin sodium
[0030] Step (1) using dean-stark trap, 20g ethyl 7-bromooxyheptanoate, 12g(s)-2,2-dimethylcyclopropanecarboxamide and 0.2g p-toluenesulfonic acid were dissolved in toluene solution 120 Under the condition of reflux and water separation for 24 hours, after the condensation was completed, the reaction mixture was washed with dilute hydrochloric acid and a saturated aqueous solution of sodium bisulfite, and then concentrated to recover toluene. The viscous was hydrolyzed in 15ml of ethanol and 50ml of 30% sodium carbonate aqueous solution for 8 hours, adjusted to pH 3.0-4.0 with concentrated hydrochloric acid, extracted twice with 25ml of ethyl acetate, and concentrated to obtain 22.8g of viscous.
[0031] Step (2) Stir and dissolve the sticky substance in the previous step, 15g of cysteine hydrochloride in 100ml of water and 20ml of ethanol, add dropwise 20ml of 50% sodium hydroxide solution, and stir at room temperatu...
Embodiment 3
[0035] Step (1) 15g ethyl 7-chlorooxyheptanoate, 8.2g(s)-2,2-dimethylcyclopropanecarboxamide and 0.25g p-toluenesulfonic acid were dissolved in xylene solution at a temperature of 130°C Reflux for water separation reaction. After the condensation is completed, the reaction mixture is washed with dilute hydrochloric acid and saturated aqueous sodium bisulfite solution, and then concentrated to recover xylene. The viscous was hydrolyzed in 15ml of ethanol and 15ml of 50% sodium hydroxide solution, adjusted to pH 3-4 with 2N hydrochloric acid solution, extracted twice with 20ml of ethyl acetate, and concentrated to obtain 19.8g of viscous.
[0036] Step (2) Add 12g of cysteine hydrochloride and 80ml of 2N sodium hydroxide solution to the sticky substance in the previous step, and stir at room temperature for 13h;
[0037] Step (3) Use hydrochloric acid to adjust the pH of the solution to 2.0, wash 3 times with 50ml of petroleum ether, heat up to 90°C, and then keep warm for 1h;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com