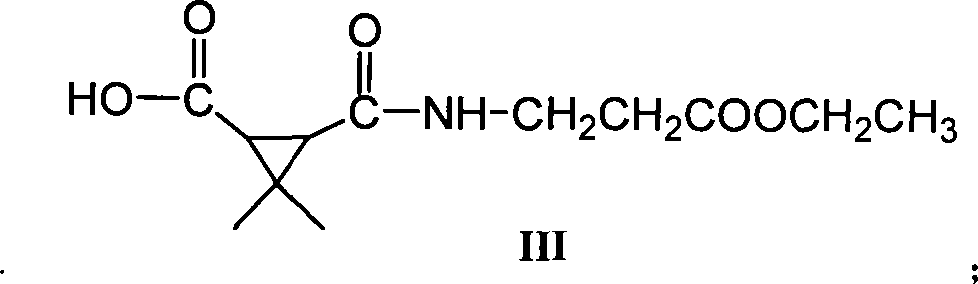Method for synthesizing pyrethroid hapten compounds
A technology of pyrethroids and synthetic methods, which is applied in the field of synthesis of pyrethroid hapten compounds, and can solve problems such as inability to obtain target compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
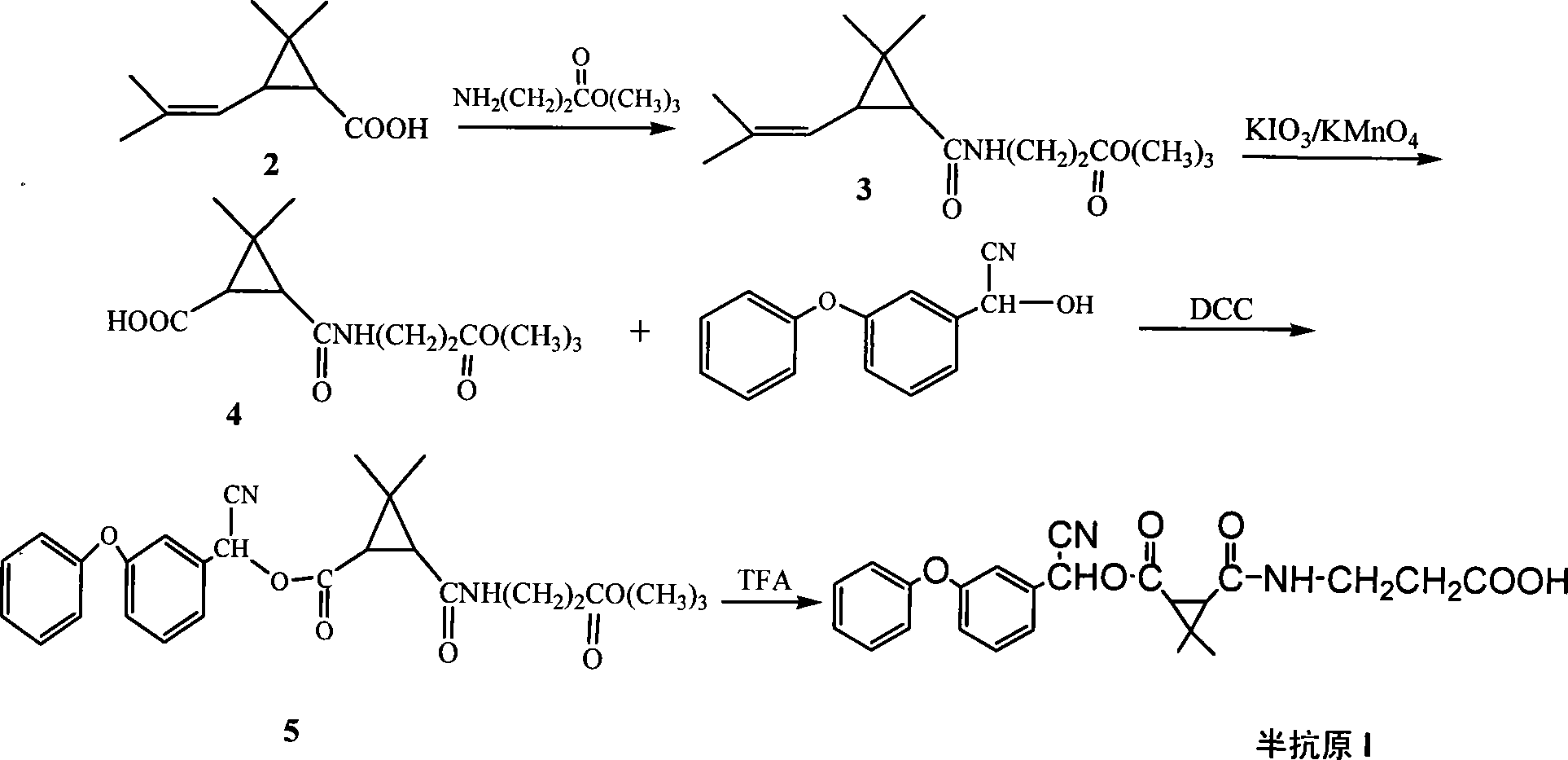
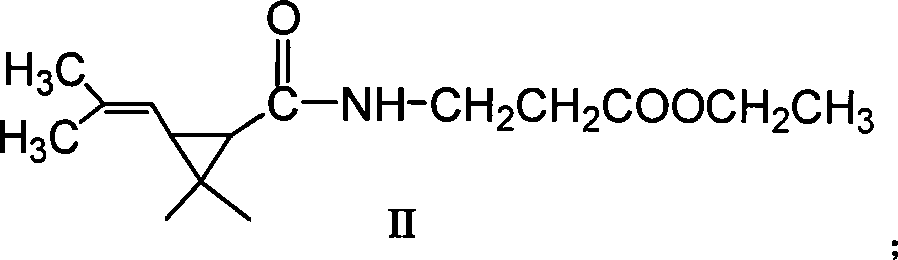
[0041] The preparation of embodiment 1,3-(2,2-dimethyl-3-(2'-methacryl) cyclopropanecarbonamido) ethyl propionate (molecular structural formula is II, hereinafter referred to as II):
[0042] Add 1.582g (11mmol) γ-aminobutyric acid ethyl ester hydrochloride, 0.178g (1.45mmol) DMAP, 1.547g (15.3mmol) triethylamine and 120mL chloroform in the reaction bottle, add dropwise under ice-salt bath 1.28g (6.9mmol) of dimethyl chrysanthemum acid chloride and 30mL of chloroform were added dropwise, and reacted at 20°C for 6h. The reaction solution was washed three times with dilute hydrochloric acid, and then washed with water until neutral, anhydrous Na 2 SO 4 It was dried and concentrated to obtain 1.96 g of crude product, which was purified by silica gel column to obtain 1.71 g (6.7 mmol) of the product, Y=96.9%.
Embodiment 2
[0043] Embodiment 2, the preparation of 3-(3-ethoxy-3-oxopropanecarbonyl)-2,2-dimethylcyclopropanecarboxylic acid (molecular structural formula is III, hereinafter referred to as III):
[0044] Add 2.645g (10.3mmol) II, 0.166g (0.61mmol) ruthenium trichloride hydrate, 8.39g (39mmol) sodium periodate and 70mL (CCl 4 :CH 3 CN:H2O=10:10:15), the air was cut off and the reaction was stirred under reflux for 24h. After the reaction solution was cooled, it was filtered and the filter cake was washed with saturated sodium chloride solution, and the filtrate was extracted three times with dichloromethane, and anhydrous Na 2 SO 4 Dry, concentrate and wash with saturated NaHCO 3 Extract, adjust to pH = 2 with hydrochloric acid after liquid separation, extract with ethyl acetate, anhydrous Na 2 SO 4 It was dried, concentrated, and purified by a silica gel column to obtain 1.78 g (7.2 mmol) of the product, Y=69.7%.
Embodiment 3
[0045] The preparation of embodiment 3,2-hydroxyl-2-(3-phenoxyphenyl) acetonitrile:
[0046] After adding 4.72g (24.8mmol) sodium bisulfate and 20mL water in the reaction flask, add 4.90g (24.8mmol) m-phenoxybenzaldehyde dropwise, the dropping temperature is 20°C, and the dropping time is 40min; Continue to react at 20° C. for 3 h after completion, and white insoluble matter is produced after the reaction is completed.
[0047] Then, a solution obtained by dissolving 2.11 g (43.1 mmol) of NaCN in 20 mL of water was added dropwise to the above-mentioned white insoluble matter, and the dropping time was 30 min. After the dropwise addition was completed, the stirring reaction was continued at 15° C. for 3 h.
[0048] After the above reaction was completed, the reaction product was extracted with dichloromethane, and after washing with water, anhydrous Na 2 SO 4 After drying, the dichloromethane solution of the product 2-hydroxy-2-(3-phenoxyphenyl)acetonitrile was obtained, whi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com