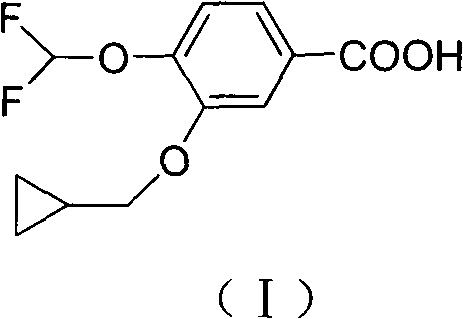
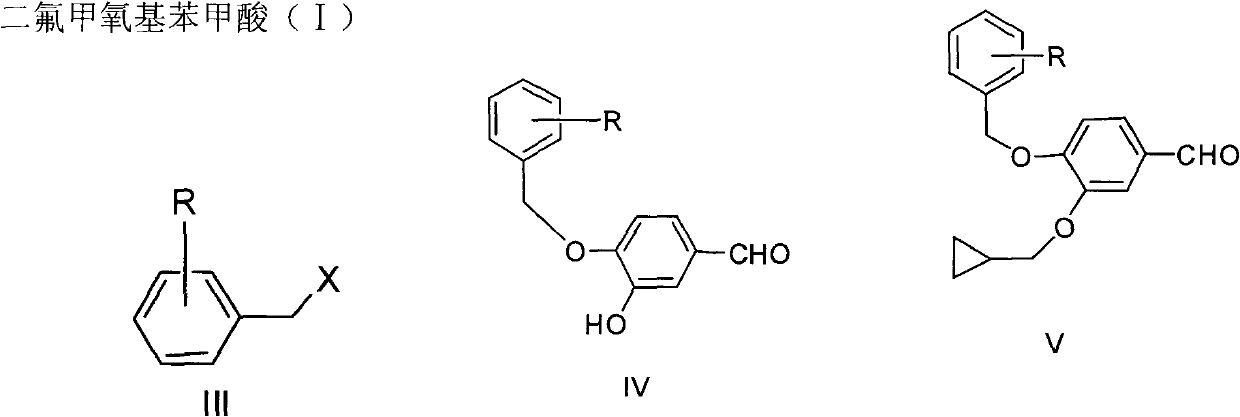
Preparation method for roflumilast intermediate
A technology of roflumilast and intermediates, which is applied in the preparation of organic compounds, the preparation of carboxylate, chemical instruments and methods, etc., can solve the problems of severe reaction, large consumption of raw materials, poor reaction selectivity, etc., and achieve mild reaction conditions. , post-processing simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 4
[0030] The preparation of embodiment 4-benzyloxy-3-hydroxybenzaldehyde (IV):
[0031] Add acetone (600ml), 3,4-dihydroxybenzaldehyde (II) (60.0g, 434mmol) in the reaction flask, benzyl bromide (74.0g, 434mmol), anhydrous potassium carbonate (90.0g, 651mmol), stir, Heat to reflux for 3h, filter, concentrate the filtrate under reduced pressure, add ethyl acetate (600ml), extract with 1N NaOH aqueous solution (600ml×2), combine the aqueous layers, neutralize with 1N hydrochloric acid (1200ml), add ethyl acetate (600ml), organic The layer was washed with saturated disodium hydrogen phosphate aqueous solution (300ml×2), dried over anhydrous sodium sulfate, filtered, concentrated and solidified, and recrystallized from ethanol to obtain 67.5g of light yellow crystals, yield 68%, mp 120~122°C. 1 H NMR (300MHz, CDCl 3 )δ: 5.84(s, 1H, OH), 7.03(d, J=8.0Hz, 1H, Ar-H), 7.37~7.45(m, 7H, Ar-H), 5.20(s, 2H, CH 2 ), 9.82 (s, 1H, CHO).
[0032] The preparation of embodiment 4-benzyloxy-3-c...
Embodiment 3
[0036] The preparation of embodiment 3-cyclopropylmethoxy-4-difluoromethoxybenzaldehyde (VII):
[0037] Add DMF (450ml), 4-hydroxyl-3-cyclopropylmethoxybenzaldehyde (V) (45g, 232mmol), potassium carbonate (64.1g, 464mmol) into the reaction flask, after heating up to 60°C, feed difluoro Chloromethane gas for 8 hours, filtered, concentrated under reduced pressure, 2N sodium hydroxide solution (500ml) was added to the residue, extracted with dichloromethane (400ml×2), the organic layers were combined, dried over anhydrous sodium sulfate and filtered, the filtrate Concentrate under reduced pressure to obtain 53.8 g of light yellow transparent liquid with a yield of 96%.
Embodiment 3
[0038] The preparation of embodiment 3-cyclopropylmethoxy-4-difluoromethoxybenzoic acid (I):
[0039] At 65°C, 30% hydrogen peroxide (103ml, 1030mmol) was added into a mixture of 3-cyclopropylmethoxy-4-difluoromethoxybenzaldehyde (VI) (50g, 207mmol), 50% potassium hydroxide (60.6ml, 827mmol ) and methanol (500ml), stirred for 1h. After the reaction, water (1.5 L) was added, and concentrated hydrochloric acid was added to adjust the pH to 2. After suction filtration and drying of the filter cake, 51.5 g of off-white solid was obtained, yield 96%, mp 118-120°C. ESI-MS (m / z): 257[M-H]-; 1 H NMR (300MHz, CDCl 3 )δ: 0.34 ~ 0.36 (m, 2H, CH 2 C H 2 CH), 0.62~0.68(m, 2H, CH 2 C H 2 CH), 1.28(m, 1H, CH), 3.90(d, J=6.7Hz, 2H, CHC H 2 O), 6.71(t, 1H, J=75.0Hz, CHF 2 ), 7.18 (d, J=7.2Hz, 1H, Ar-H), 7.62-7.64 (m, 2H, Ar-H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com