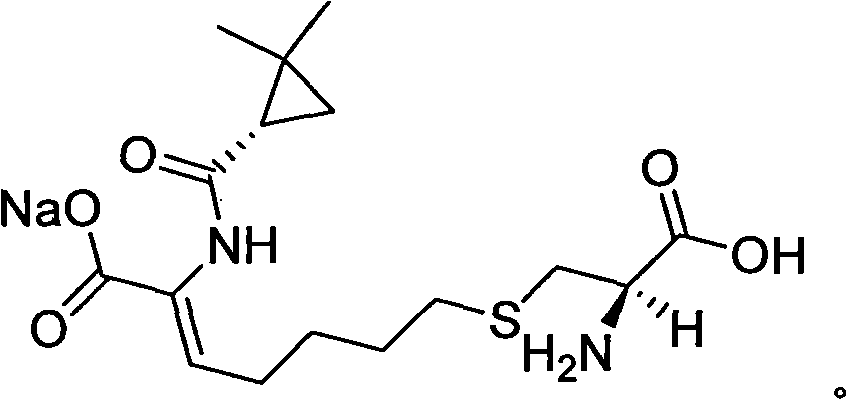
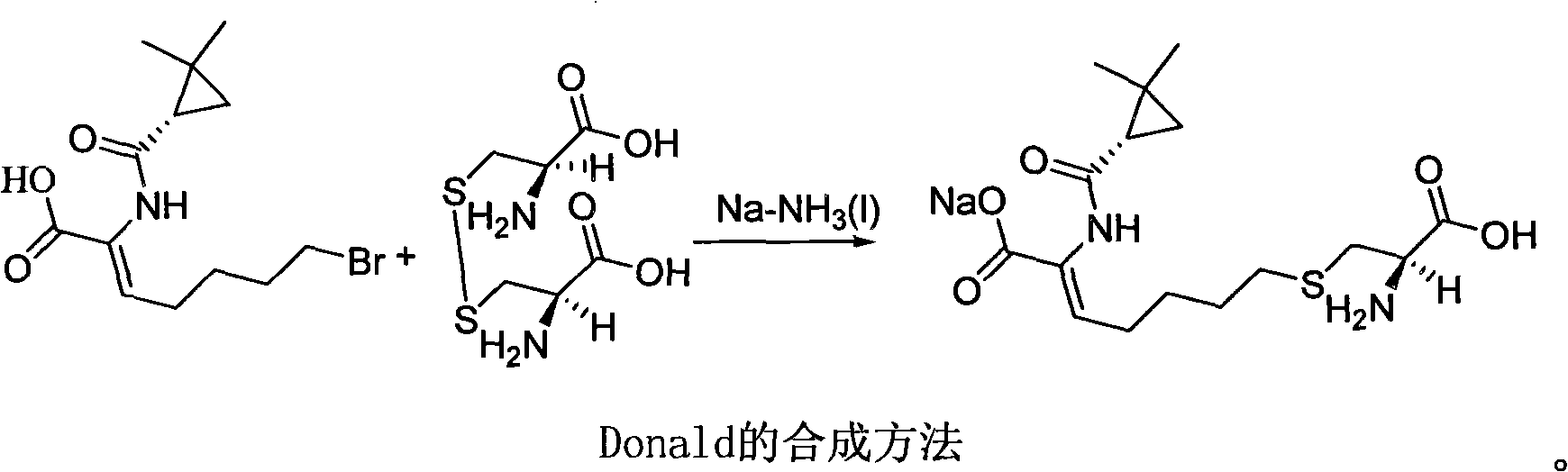
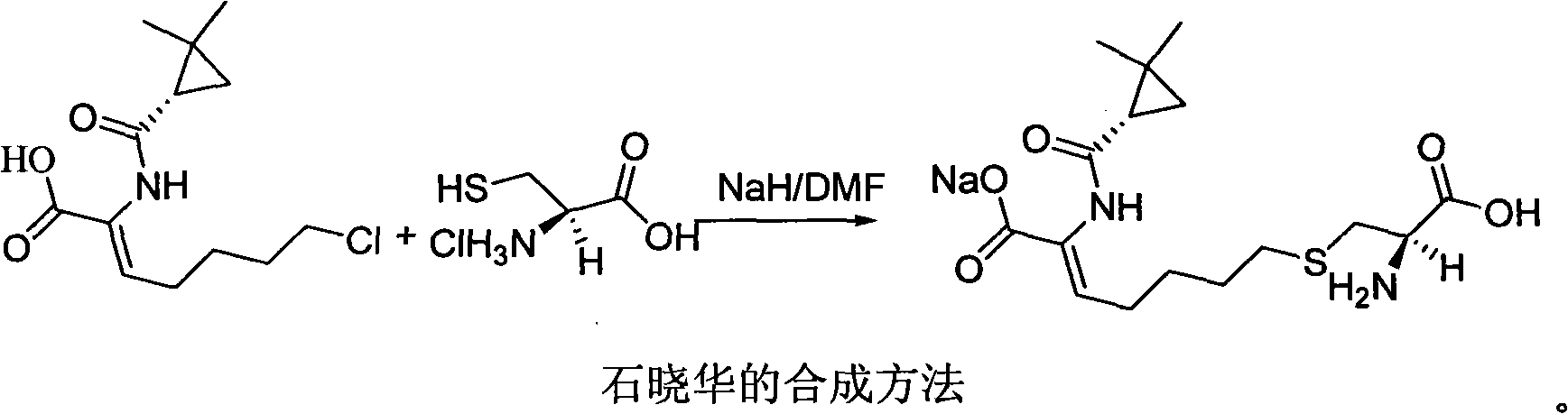
Method for synthesizing cilastatin sodium
A technology of cilastatin sodium and a synthesis method, which is applied in the field of drug synthesis, can solve the problems of complicated post-processing process of cilastatin sodium, requires large solvent, pollutes the environment, etc., and achieves easy control of operation process and high optical purity. , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation of embodiment 1 (Z)-7-chloro-2-((1S)-2,2-dimethylcyclopropanecarboxamido)-2-heptenoic acid ethyl ester
[0043] Dissolve 1.1g (S)-2,2-dimethylcyclopropanecarboxamide, 2g ethyl 7-chloro-2-oxoheptanoate and 0.01g p-toluenesulfonic acid in 50mL toluene, heat to reflux, and separate water, the reaction was completed, toluene was evaporated, washed with water, washed with saturated brine, dried, and spin-dried to obtain a crude product, which was purified to obtain a light yellow oil (Z)-7-chloro-2-((1S)-2, 2-Dimethylcyclopropanecarboxamido)-2-heptenoic acid ethyl ester, yield 67%.
Embodiment 2
[0044] The preparation of embodiment 2 cilastatin dialkyl ester
[0045] Add 0.5 g of (Z)-7-chloro-2-((1S)-2,2-dimethylcyclopropanecarboxamido)-2-heptenoic acid prepared in Example 1 in a 100 mL round bottom flask Ethyl ester (1.66mmol), 0.25gNaI (1.66mmol) and 45mL acetone, heated to reflux for 12h, after the reaction, filtered, and the filtrate was spin-dried to obtain (Z)-7-iodo-2-((S)-2,2 -Dimethylcyclopropanecarboxamido)-2-ethyl heptenoate, directly used in the next step reaction.
[0046] Under a nitrogen atmosphere, add 0.22 g of the above-mentioned (Z)-7-iodo-2-((S)-2,2-dimethylcyclopropanecarboxamido)-2-heptenoic acid ethyl ester (0.56mmol) and 0.1g (0.56mmol) cysteine methyl ester hydrochloride, 0.36g K 3 PO 4 (1.7mmol) and 40mL tetrahydrofuran (THF), react in ultrasonic for 15min, place the reaction device in an oil bath heated to 80°C for reflux for 12h, after the reaction, cool to room temperature, evaporate the solvent under reduced pressure, and It was dis...
Embodiment 3~4
[0050] The preparation of embodiment 3~4 cilastatin dialkyl ester
[0051] In addition to CsCO 3 or K 2 HPO 4 Replace K 3 Except for PO4, other operations were the same as in Example 2 to prepare cilastatin dialkyl ester.
[0052] Investigate the impact of different bases on the cilastatin dialkyl ester yield under the same conditions in Table 1:
[0053] The kind of table 1 alkali is on the impact of cilastatin dialkyl ester productive rate
[0054]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com