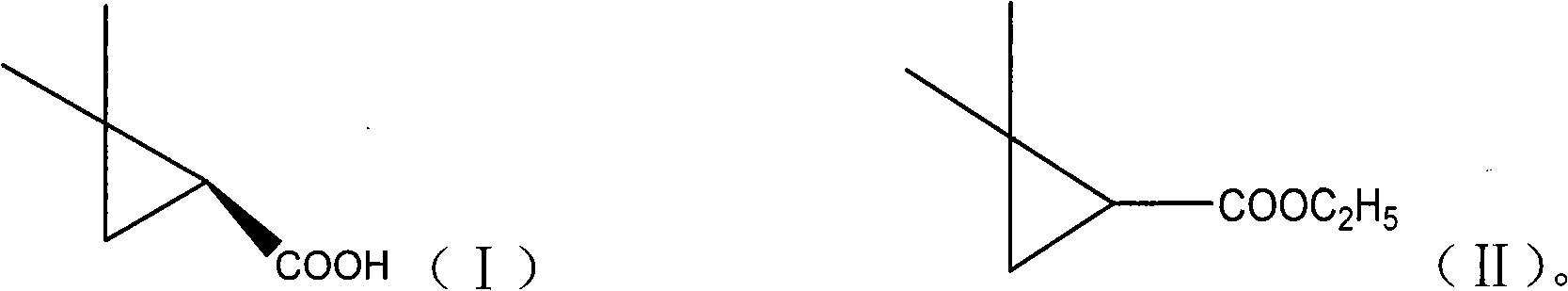Preparation method of (S)-(+)-2,2-dimethylcyclopropanecarboxylic acid by biocatalysis
A technology of ethyl dimethylcyclopropanecarboxylate and dimethylcyclopropane is applied in the field of biocatalytic preparation of (S)-(+)-2,2-dimethylcyclopropanecarboxylic acid, and can solve the problem of low yield, Problems such as many process steps, to achieve the effects of high catalytic efficiency, reduced optical purity, and no by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of (S)-(+)-2,2-dimethylcyclopropanecarboxylic acid
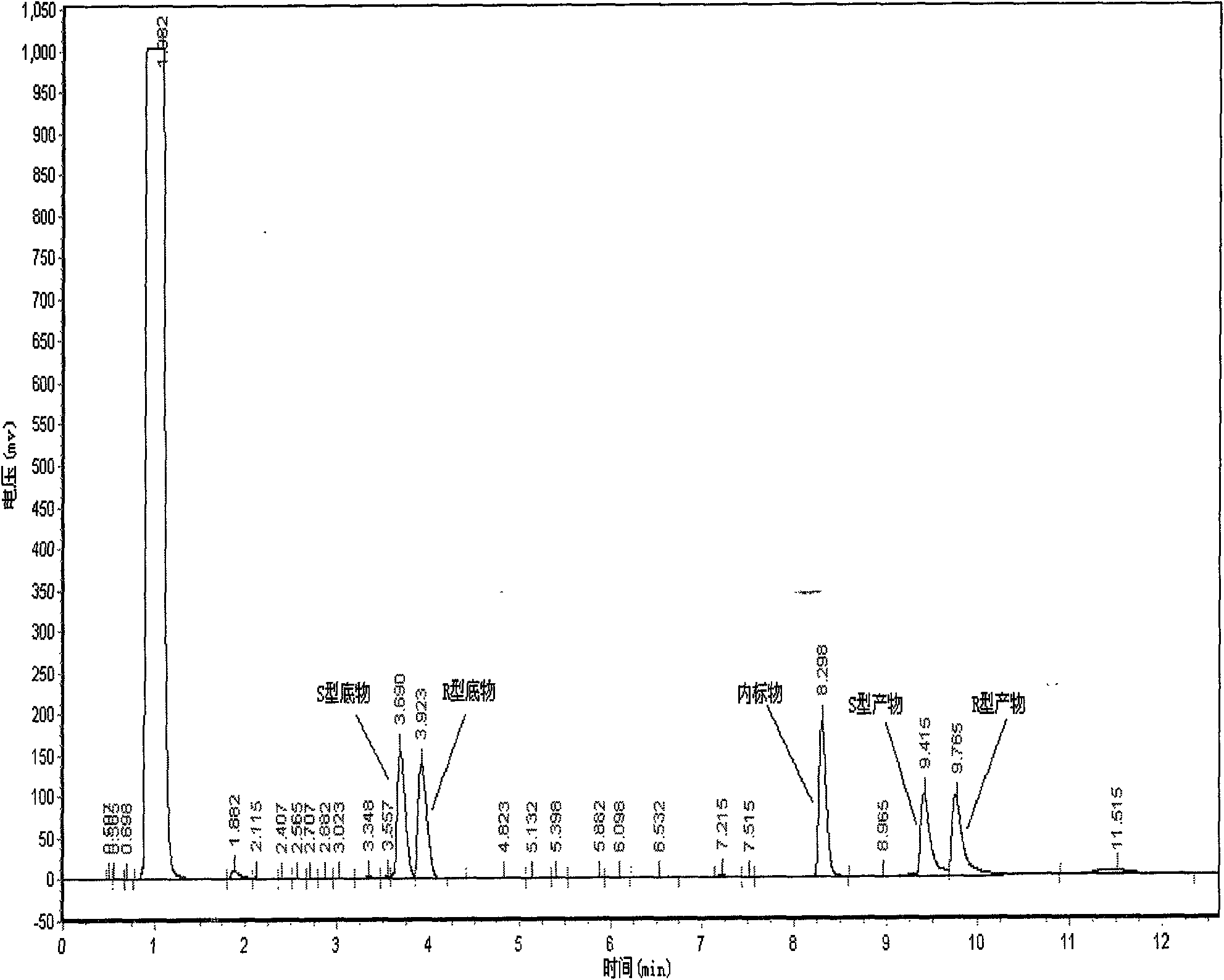
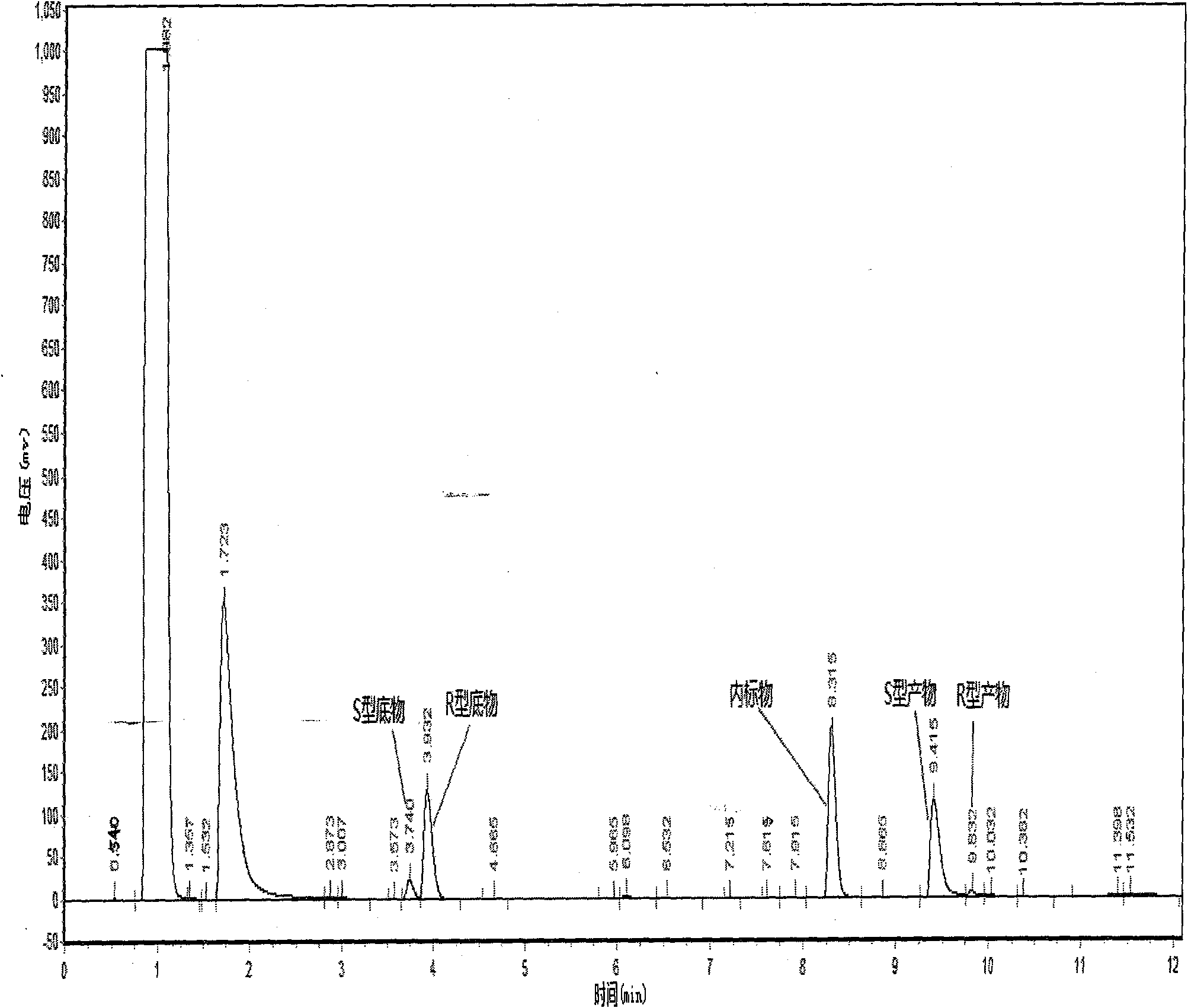
[0037] Take three parts of 150mg Novozym 435 lipase in parallel and place in 25ml Erlenmeyer flasks, add 10ml pH of 7.0 phosphate buffer respectively, then add 0.25mmol substrate 2,2-dimethylcyclopropane ethyl carboxylate for different Symmetrical hydrolysis reaction. The reaction conditions are: the rotating speed of the shaker is 200r / min, the transformation temperature is 30°C, and the transformation time is 40h. After the reaction finishes, combine three parts of reaction solutions, get 10ml reaction solution and extract twice with equal volume ethyl acetate, settle to 20ml, analyze with gas chromatography, as figure 2 shown. The calculation of the yield adopts the internal standard method, and the e.e. value and yield of the product are 94.65% and 38.36%, respectively. The remaining reaction solution was separated as follows: adjust the pH of the reaction solution to 9.0 with a N...
Embodiment 2~7
[0039] With reference to the method for embodiment 1, add the substrate of different concentrations listed in table 1, other conditions are identical, shaker speed 200r / min, investigate the influence of substrate concentration on asymmetric hydrolysis reaction, substrate concentration in table 1 is every Add the millimoles of ethyl 2,2-dimethylcyclopropanecarboxylate to 1 liter of phosphate buffer, the enzyme concentration is the number of grams of Novozym 435 lipase added per 1 liter of phosphate buffer, and the pH is the initial pH of the phosphate buffer , the conversion time in the table is the reaction time, and the conversion temperature is the reaction temperature. After the reaction finished, the reaction solution was extracted twice with equal volume of ethyl acetate and then settled to 20ml, and analyzed by gas chromatography, the results are shown in Table 1:
[0040] Table 1
[0041]
Embodiment 8~14
[0043] With reference to the method of Example 1, the concentration of adding Novozym 435 lipase in the reaction system is different, specifically as listed in Table 2, other conditions are the same, the shaking table speed is 200r / min, and the effect of enzyme concentration on 2,2-dimethylcyclo The impact of propane ethyl formate asymmetric hydrolysis reaction, the substrate concentration in table 2 is the millimole of 2,2-dimethylcyclopropane ethyl carboxylate per 1 liter of phosphate buffer, and the enzyme concentration is per 1 liter of phosphate buffer Add the grams of Novozym 435 lipase to the solution, and the pH is the initial pH of the phosphate buffer. After the reaction finished, the reaction solution was extracted twice with equal volume of ethyl acetate and then settled to 20ml, and analyzed by gas chromatography, the results are shown in Table 2:
[0044] Table 2
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com