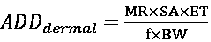Patents
Literature
44 results about "3-Dichloro-2-propanol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
1,3-Dichloro-2-propanol is a food processing contaminant. Biotransformation of 1,3-dichloro-2-propanol to epichlorohydrin by the whole cells of recombinant Escherichia coli via resin-based in situ product removal has been investigated.
Method for continuously preparing epichlorohydrin by glycerine reaction fractional distillation
InactiveCN101337950AEfficient separationGuaranteed purityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsBiodiesel1-Propanol
The invention relates to a method for preparing epoxy chloropropane which is obtained through successive reaction and distillation coupling technology after glycerine that is the by-product of biological diesel oil reacts with chlorine hydride through substitution reaction under homogeneous multielement catalysis and further performs saponification cyclization reaction. The method comprises the following steps: (1) chlorine hydride is pumped in glycerine that is the by-product of biological diesel oil for reaction under homogeneous multielement catalysis, and the mixture composed of 1, 3-dichloro-2-propanol and 2, 3-dichloro-1-propanol is prepared through the technologies of continuous feeding, continuous catalyzed chlorination and continuous rectification; (2) the isomeride mixture solution of 1, 3-dichloro-2-propanol and 2, 3-dichloro-1-propanol performs the saponification cyclization to generate a product of epoxy chloropropane in alkaline solution. The method has the advantages of mild reaction condition is mild, high catalyst activity, dedicated catalytic performance, high product selectivity, process 'cleaning', easy separation, continuous operation, environment-friendliness, etc.
Owner:JIANGSU POLYTECHNIC UNIVERSITY
Method for preparing ganciclovir
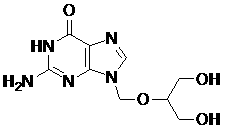
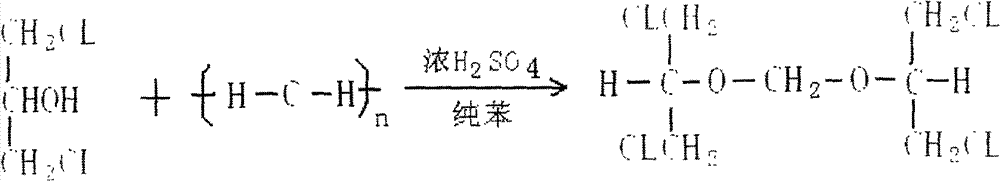
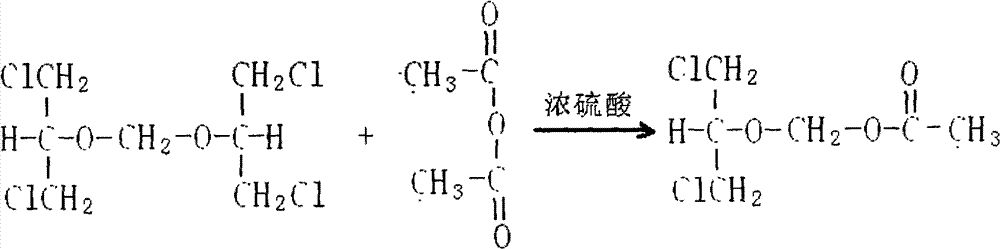
The invention relates to a method for preparing ganciclovir. The method comprises the following steps of: a, adding paraformaldehyde into 1,3-dichloro-2-propanol [2], reacting under the action of a catalyst to obtain hemiformal [3], and reacting with an acetic anhydride to obtain 1,3-dichloro-2-acetoxylmethoxylpropane [4]; b, making 1,3-dichloro-2-acetoxylmethoxylpropane [4] react with absolute postassium acetate or anhydrous sodium acetate in an organic solvent medium in the presence of a tetraalkyl ammonium bromide catalyst with 1-20 carbon atoms and an acetic anhydride serving as a dehydrating agent to obtain 1,3-diacetoxy-2-acetoxymethoxyl propane [5]; c, performing a condensation reaction on 1,3-diacetoxy-2-acetoxymethoxyl propane [5] and 2,9-diacetyl guanine [6] in an organic solvent medium in the presence of a catalyst and an acetic anhydride serving as a dehydrating agent to obtain triacetyl ganciclovir [7]; and d, hydrolyzing the triacetyl ganciclovir [7] to obtain ganciclovir [1]. The method has the advantages of easy and controllable preparation process, high utilization ratios of raw materials, low cost and high yield of a prepared ganciclovir product.
Owner:HUBEI GEDIAN HUMANWELL PHARMACEUTICAL CO LTD
Method for detecting volatile organic chlorides in resin by using headspace gas chromatography
ActiveCN107064339AGood precisionNo treatment neededComponent separationMatrix solutionGas liquid chromatographic
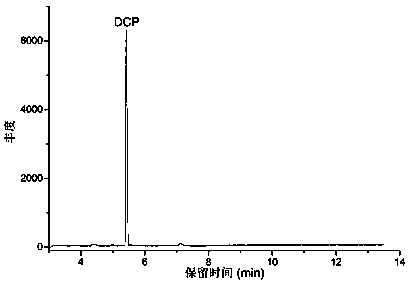
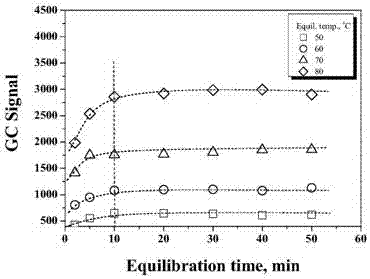
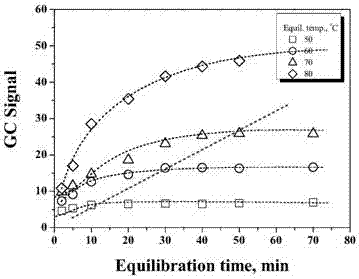
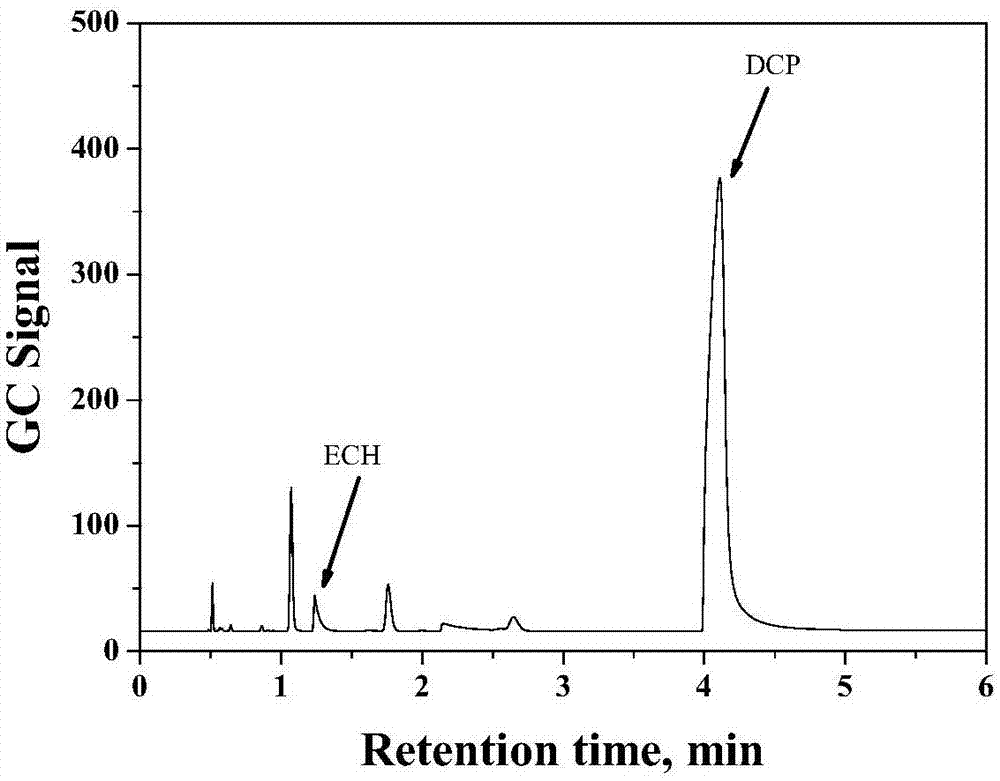
The invention discloses a method for detecting volatile organic chlorides in resin by using headspace gas chromatography. The method specifically is a headspace gas chromatography (HS-GC) method for detecting residual epichlorohydrin (ECH) and a by-product, 1,3-dichloro-2-propanol (DCP), in a polyamide polyamine epichlorohydrin (PAE) solution. The method is based on a phase balance achieved by a closed headspace bottle before GC detection under the headspace conditions of 60 DEG C and 30 minutes. An experiment proves that a PAE matrix solution affects the phase balance of a to-be-detected substance. Therefore, the accuracy of the method is tested by adopting a standard addition method. The result shows that the method has relatively good precision (RSD is smaller than 2.90%) and accuracy (the recovery rate range is 93.6%-105%). Therefore, the method is suitable for analysis of volatile organic chlorides in a PAE resin solution.
Owner:SOUTH CHINA UNIV OF TECH
Method for producing epoxy chloropropane by dichloropropanol
The invention provides a method for producing epoxy chloropropane by dichloropropanol, which comprises the following steps of: carrying out saponification reaction on mixture of 1, 3-dichloro-2-propanol and 2, 3-dichloro-1-propanol and sodium hydroxide under alkaline condition, wherein the operation pressure is complete vacuum, to generate epoxy chloropropane and sodium chloride, wherein the dichloropropanol is obtained by reaction between glycerol and chlorine hydride-containing chlorinating agent, the reaction rate of the 1, 3-dichloro-2-propanol is relatively quicker than that of the 2, 3-dichloro-1-propanol, and the weight of the 1, 3-dichloro-2-propanol is more than 95% of the weight of the whole dichloropropanol, and adding the sodium hydroxide into the saponification reaction by three times, wherein the concentration of the sodium hydroxide needs controlling at 20%, and the device needs internally providing with a preparation device of sodium hydroxide solution. The method is high in chloropropane yield, less in the volume of aqueous effluent to be treated, energy sources-saving, and more environment-friendly.
Owner:西安汉术化学工程股份有限公司
Parvibaculum lavamentivorans ZJB 14001, halohydrin dehalogenase enzyme gene, enzyme, engineered bacterium and application
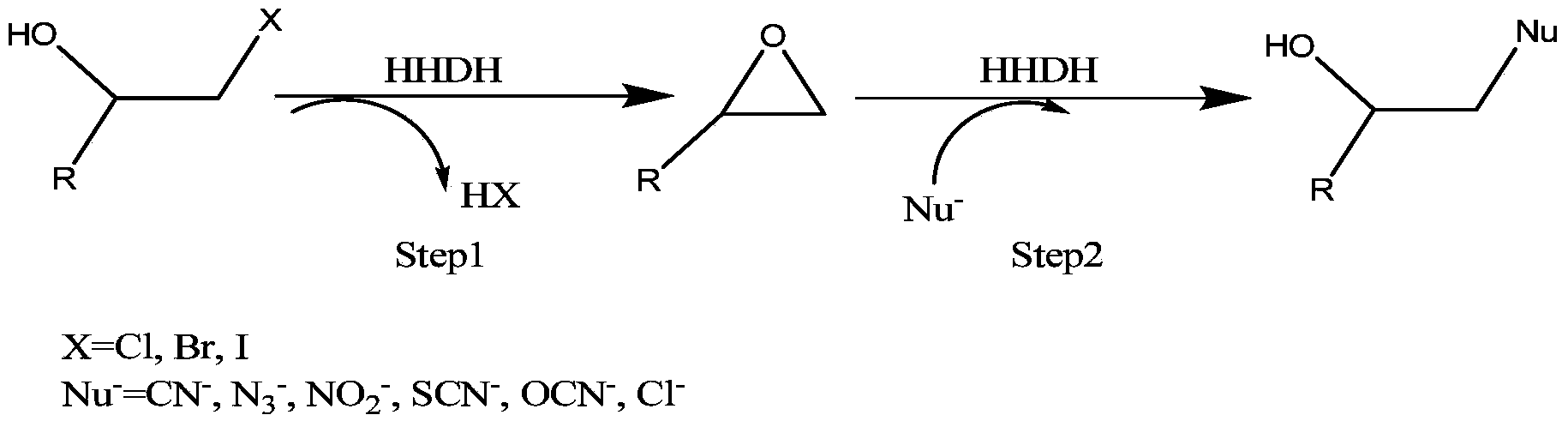
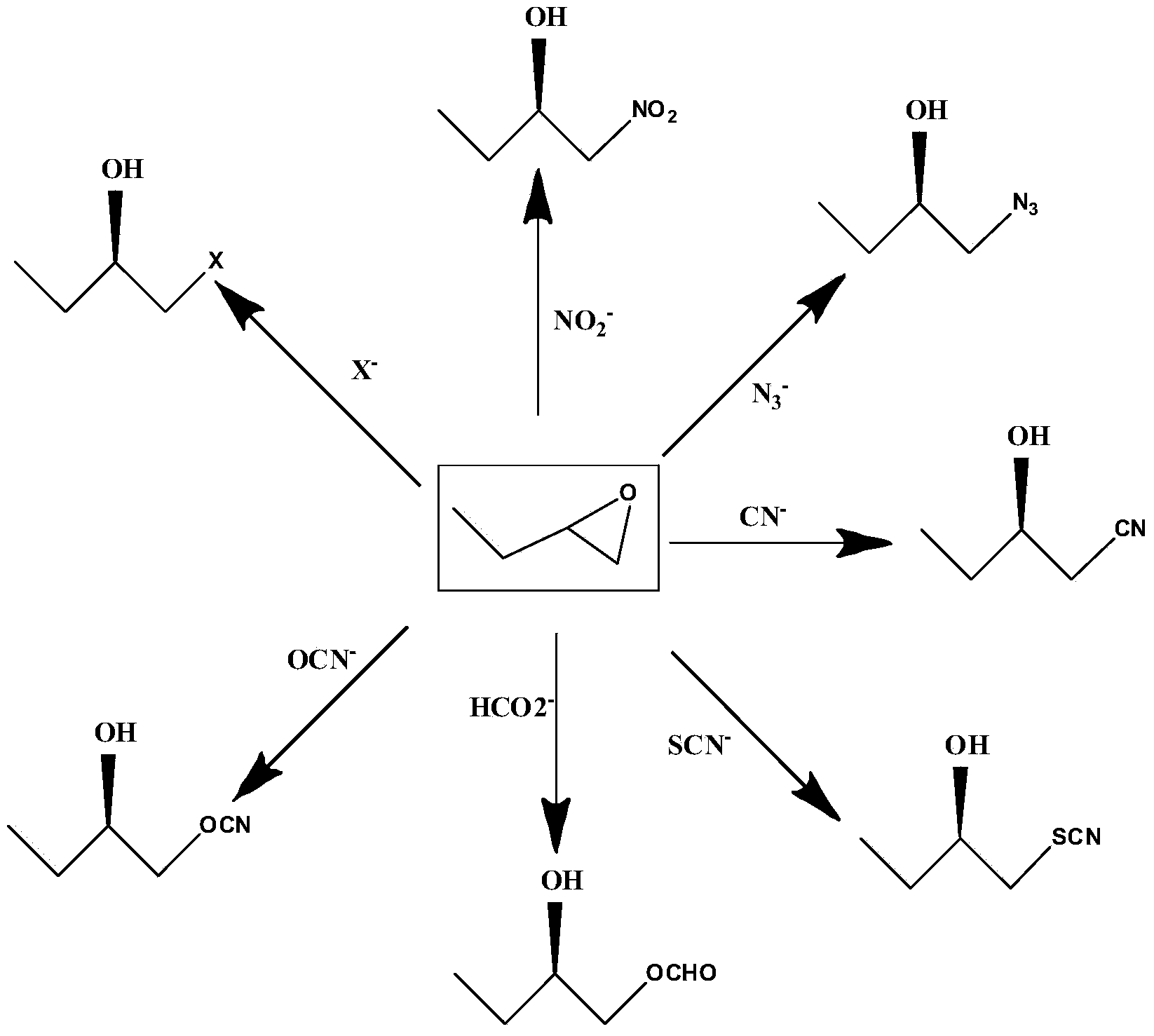
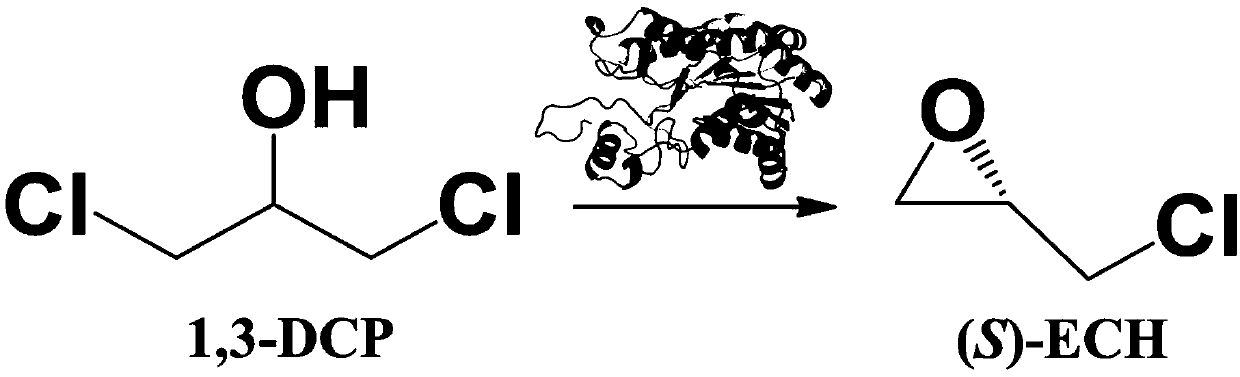
The invention provides a halohydrin dehalogenase encoded gene, a recombinant vector containing the gene, and a recombinant genetically engineered bacterium, and provides a novel bacterial strain, namely parvibaculum lavamentivorans ZJB 14001, of the gene. The recombinant halohydrin dehalogenase is regarded as an invertase to synthesize epoxy chloropropane by taking catalysis of 1,3-dichloro-2-propanol as an example, to prepare (S)-4-chloro-3-hydroxybutyronitrile by taking catalysis of CN-mediated (S)-epoxy chloropropane with a ring-opening reaction as an example, and to respectively prepare (S)-4-chloro-3-hydroxybutyronitrile and ethyl-(R)-4-cyano-3-hydroxybutyrate by taking catalysis of 1,3-dichloro-2-propanol and (S)-4-chloro-3-hydroxybutyronitrile as an example with CN-mediated ring opening.
Owner:ZHEJIANG UNIV OF TECH
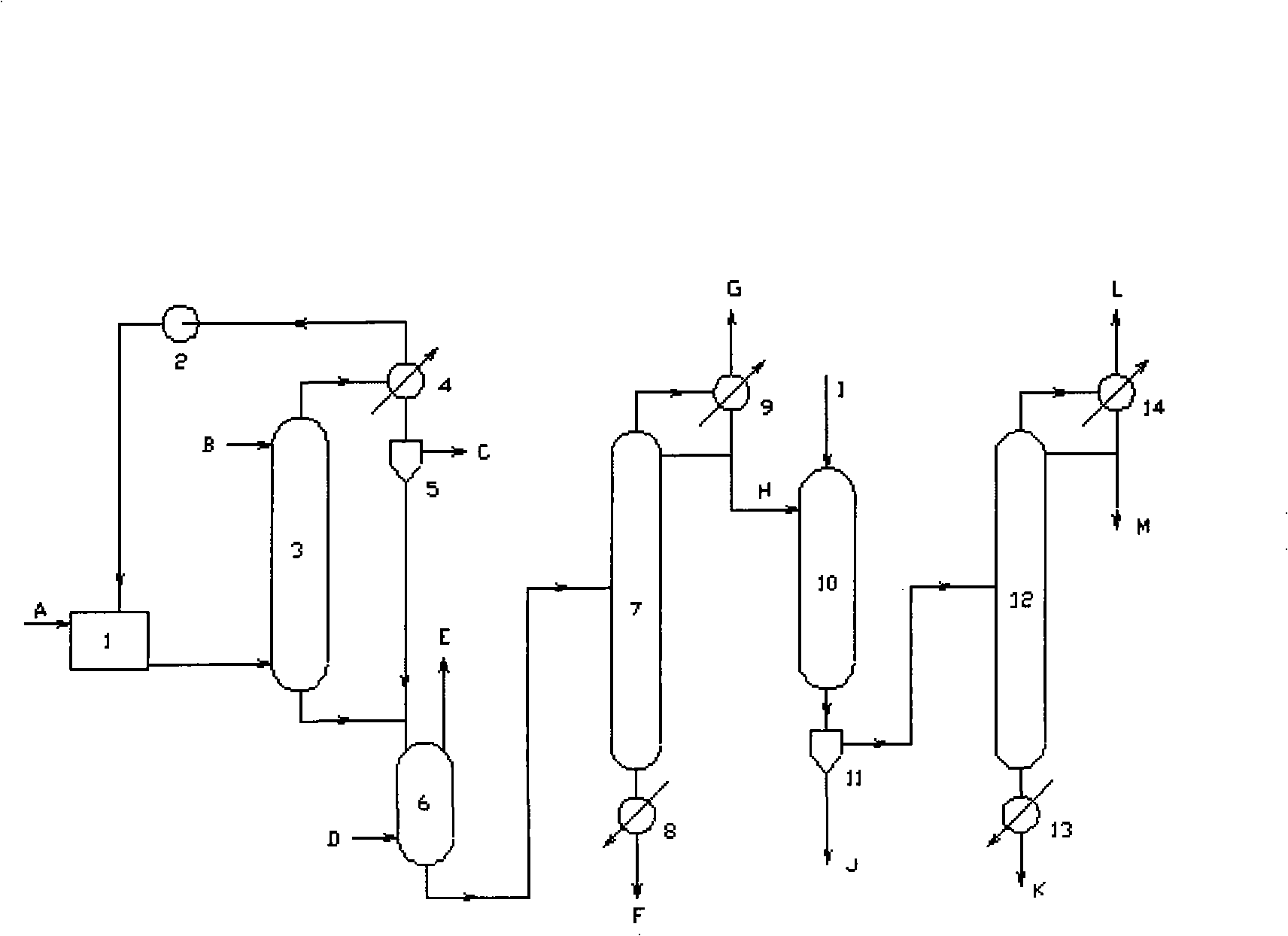
Method of preparing dichloropropanols from glycerine
ActiveUS7473809B2Increase productionOrganic compound preparationPreparation by halogen introduction1-PropanolGlycerol
Owner:SPOLEK PRO CHEMICKOU A HUTNI VYROBU
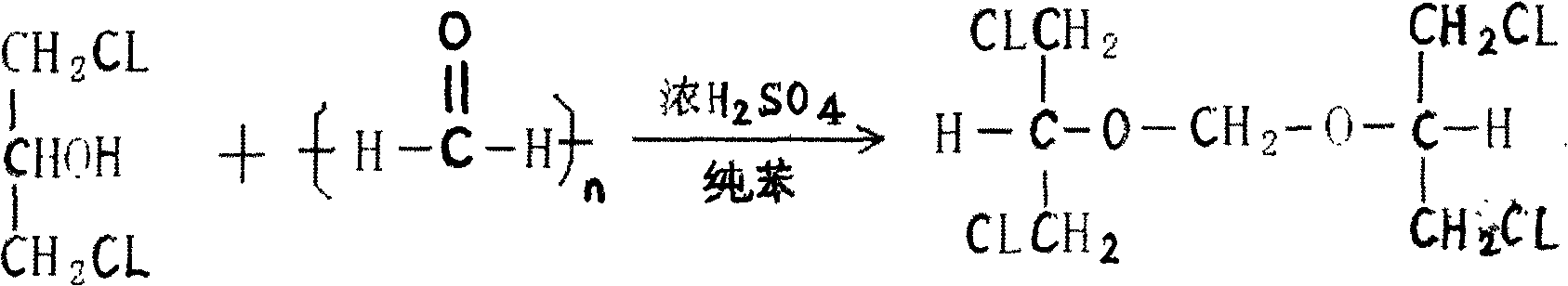
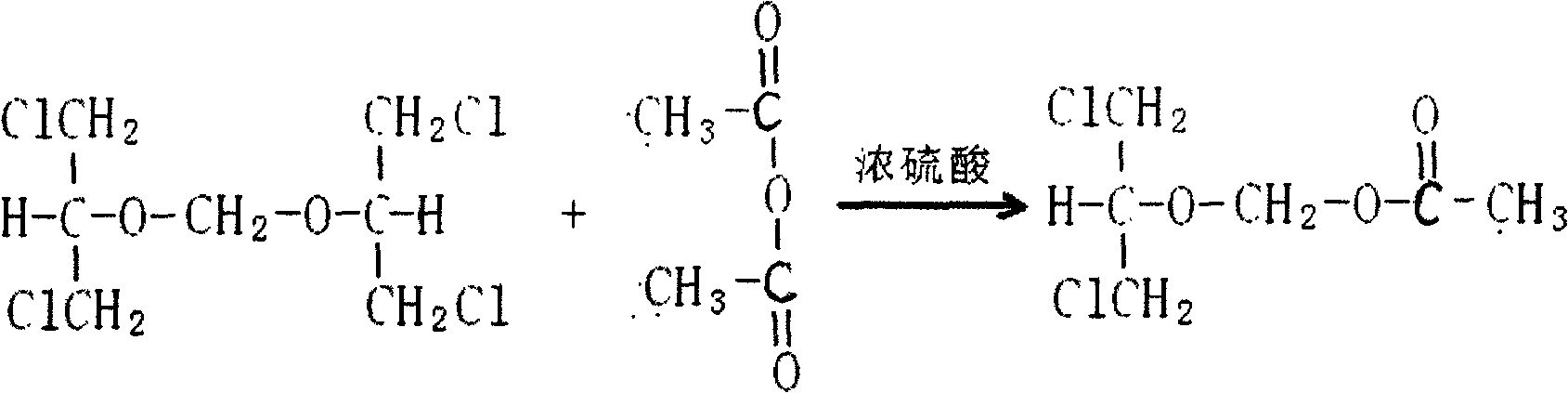
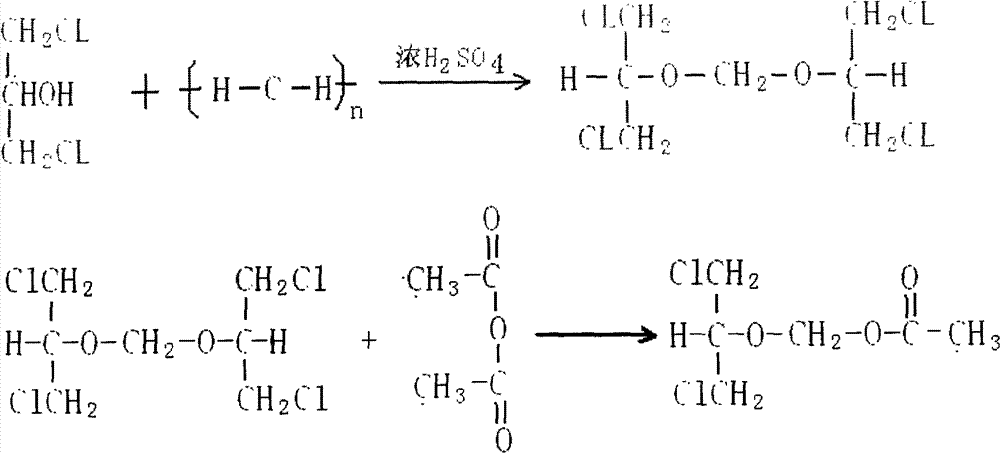
Preparation method of 2-acetoxyl group methoxy group-1.3-propylene dichloride
InactiveCN103130642AQuality improvementShort reaction timeOrganic compound preparationCarboxylic acid esters preparationAlcoholAcetic anhydride
Disclosed is a preparation method of 2-acetoxyl group methoxy group-1.3-propylene dichloride. The following substances are extracted according to weight ratio: 250kg of 1.3-dichloro-2-propyl alcohol, 60kg of paraformaldehyde, 3.68kg of vitriol, 250kg of acetic anhydride and 5kg of potassium acetate; the 1.3-dichloro-2-propyl alcohol, the paraformaldehyde and the vitriol are added to a clean and dry reaction still and are warmed slowly, the temperature rises to 96 DEG C to 98 DEG C in 2 hours, and heat preservation is carried out for 2 hours; the temperature is lowered to 10 DEG C after heat preservation, the acetic anhydride is dropped to the reaction still, and the temperature is kept between 15 DEG C and 20 DEG C; after the acetic anhydride is added, the temperature rises to 35 DEG C to 40 DEG C, and heat preservation is carried out for 10 hours; the potassium acetate is added to the reaction still, stirring is conducted, the acetic anhydride is recycled in vacuum to 100 DEG C, the temperature is lowered to 20 DEG C, and filtering is conducted; and filter liquor is pumped into a rectifying still, steamed cut fraction in a state of 115 DEG C / 10mmHg is collected, and the 2-acetoxyl group methoxy group-1.3-propylene dichloride with 80% of weight yield and more than or equal to 95% of content is obtained. According to the preparation method of the 2-acetoxyl group methoxy group-1.3-propylene dichloride, production cost is reduced, and yield is improved.
Owner:HUBEI BAOLE PHARMA
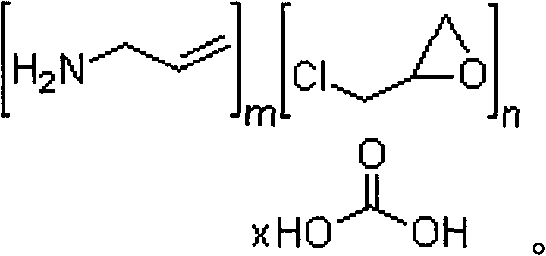
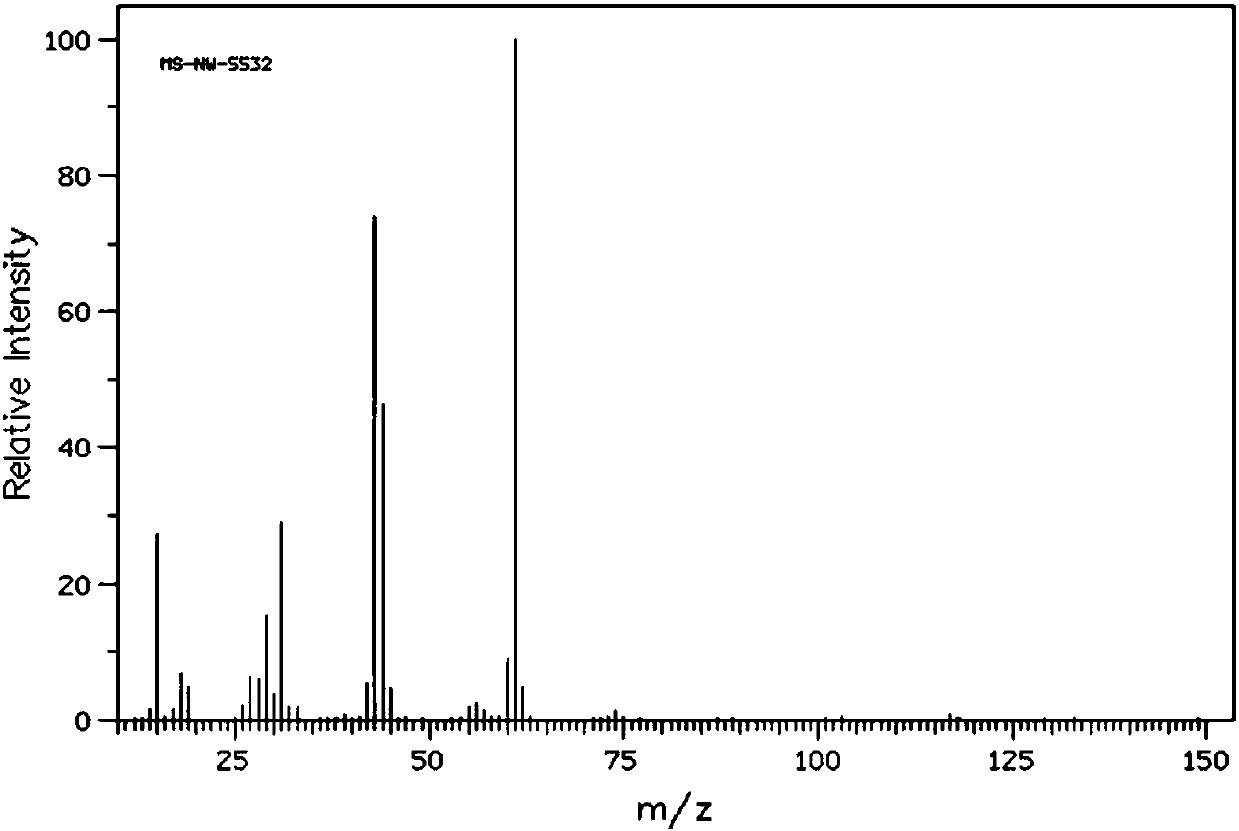
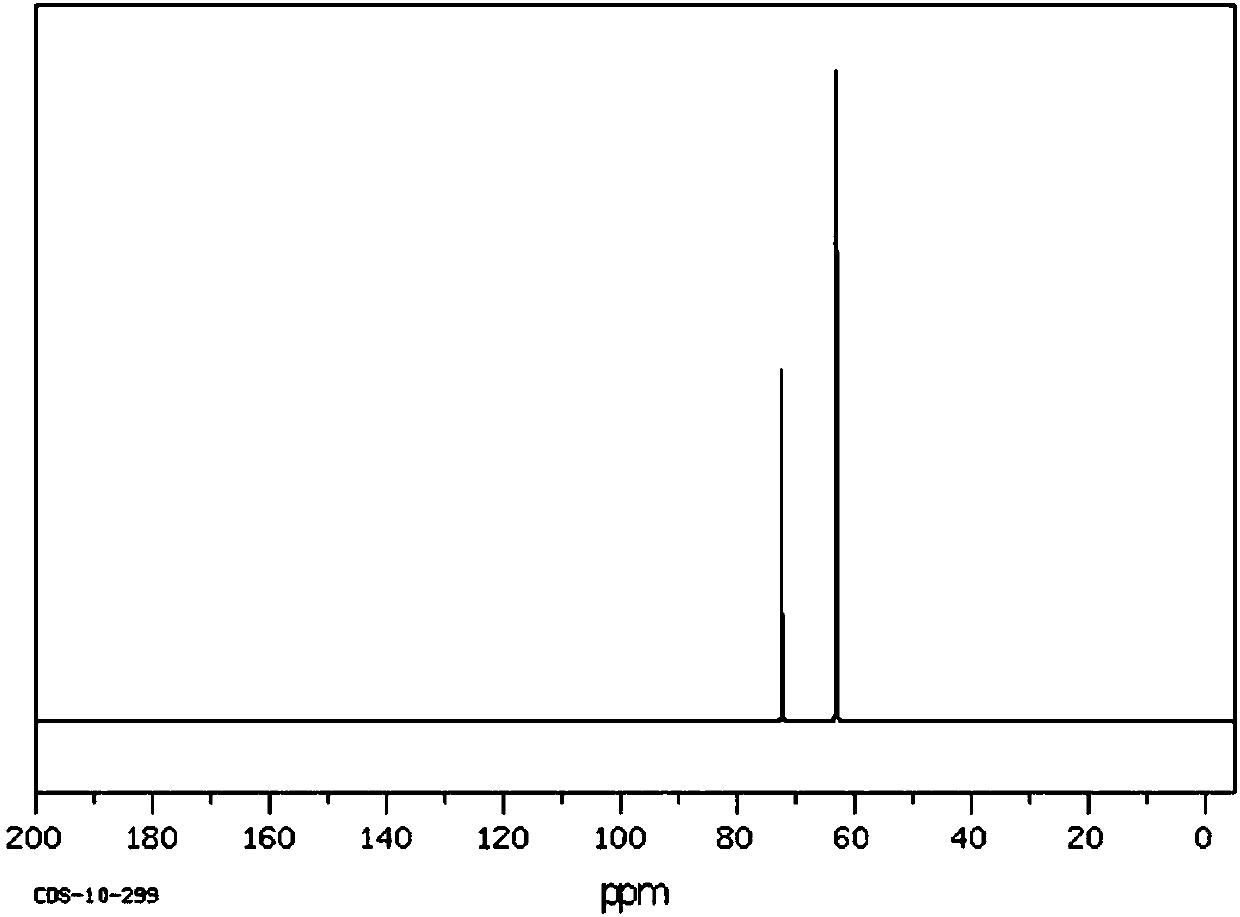
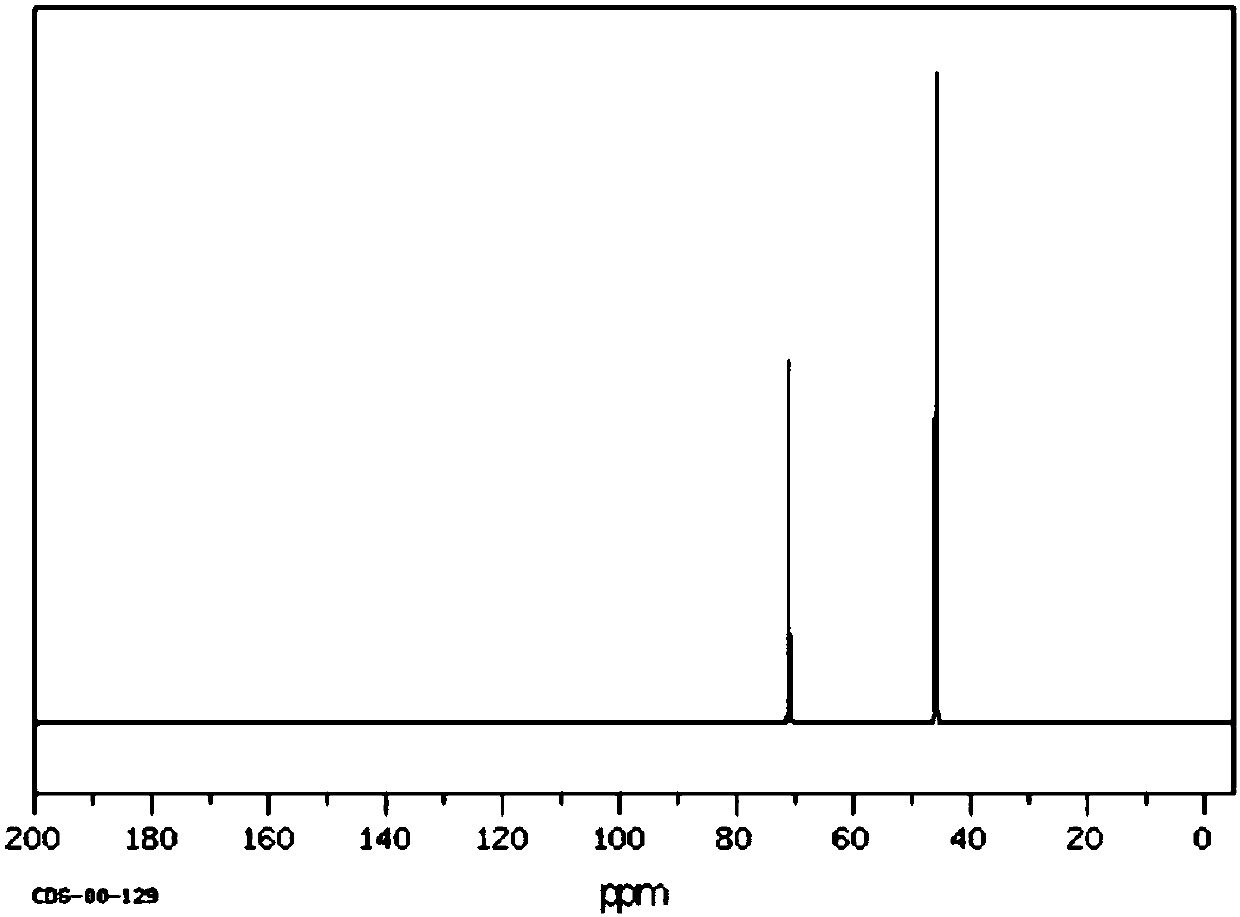
Method for preparing sevelamer carbonate
The invention provides a method for preparing sevelamer carbonate. The method comprises the following steps: a) polymerizing poly(allylamine hydrochloride) and 1, 3-dichloro-2-propanol at the temperature of between 30 and 70DEG C and the pH of 7-10, washing, and collecting the polymerized product to obtain sevelamer hydrochloride; b) dispersing the sevelamer hydrochloride in an alkali solution at the pH of 8-12 to obtain alkaline sevelamer; c) washing the alkaline sevelamer with water until neutrality to obtain an alkaline sevelamer cationic resin; and d) mixing a carbonic acid solution and the alkaline sevelamer cationic resin to obtain the sevelamer carbonate. By the method for preparing the sevelamer carbonate, the sevelamer carbonate has high purity; and in addition, by a Soxhlet extraction method, the mass transfer velocity can be improved and the reaction time is saved.
Owner:NEW FOUNDER HLDG DEV LLC +2
Preparation method of 1,3-dihydroxyacetone
ActiveCN107141208AImprove conversion rateHigh yieldOrganic compound preparationPreparation by OH group eliminationOrganic synthesisDehydrogenation
The invention relates to the technical field of organic synthesis, and discloses a preparation method of 1, 3-dihydroxyacetone. The preparation method of 1,3-dihydroxyacetone comprises the following steps: (1) carrying out contact reaction between glycerol and halogenated reagents in presence of a catalyst to prepare 1,3-dichloro-2-propanol; (2) carrying out oxidative dehydrogenation reaction on the 1,3-dichloro-2-propanol to obtain an intermediate product 1,3-dichloro-2 acetone; (3) contacting the 1,3-dichloro-2 acetone with alkali substances in a water-containing medium for hydrolysis reaction to obtain the 1,3-dihydroxyacetone, wherein a hydrolysis reaction temperature is 25 to 60 DEG C. According to the preparation method of the 1,3-dihydroxyacetone, the conversion rate of the glycerol and the yield of the 1,3-dihydroxyacetone are higher; by taking zirconium oxide as the catalyst, the preparation method disclosed by the invention is high-efficient, is low in cost and has industrial application prospect.
Owner:JIANGXI NORMAL UNIV
Method for preparing tri(1,3-dichloro-2-propyl) phosphonate
ActiveCN108997416AHigh catalytic efficiencyEasy to recyclePhosphorus organic compoundsDistillationFire retardant
The invention provides a method for preparing tri(1,3-dichloro-2-propyl) phosphonate, which relates to the technical field of a preparation method of a fire retardant. The method adopts a self-made catalyst, and uses phosphorus oxychloride and 1,3-dichloro-2-propanol as a raw material, and adopts the following steps: phosphorus oxychloride, 1,3-1,3-dichloro-2- propanol and the self-made solid acidcatalyst are added to a reaction kettle, the materials are heated to the temperature of 100-160 DEG C for 4-6 hours, the generated hydrogen chloride gas is distilled under vacuum of -0.2-0 MPa, a crude product is washed with alkali, washed with water, and is subjected to underpressure distillation to remove water. A reaction equation is shown as the specification. The raw materials of the invention are easy to obtain, the catalyst has good catalytic performance, the product has high purity, the yield is high, the reaction is mild, the operation is simple, and the product is more suitable forindustrial production.
Owner:SHANDONG TAIHE WATER TREATMENT TECH CO LTD
Halohydrin dehalogenase mutant derived from ADI (Agrobacterium radiobacter) and application of halohydrin dehalogenase mutant to preparation of (S)-epichlorohydrin
ActiveCN109593749AHigh stereoselectivitySimple reaction systemCarbon-halide lyasesGenetic engineeringAgrobacterium radiobacterPhosphate
The invention relates to a halohydrin dehalogenase mutant derived from ADI (Agrobacterium radiobacter) and an application of the halohydrin dehalogenase mutant to preparation of (S)-epichlorohydrin. The mutation site is one of the following: (1) 81st site, (2) 86th site and (3) 94th site. A plurality of mutant strains are obtained by modification of halohydrin dehalogenase, (S)-ECH prepared from 1,3-dichloro-2-propanol by conversion has extremely high stereoselectivity. In a phosphate buffer system, (S)-ECH with optical purity (e.e. value) larger than 99% can be prepared by catalysis. The technology has outstanding advantages of excellent enzyme stereoselectivity, simple reaction system, easy-to-control operation process, low production cost and the like, and has good industrial application value.
Owner:ZHEJIANG UNIV OF TECH
Preparation method of strong-acid cation-exchange resin
The invention discloses a preparation method of strong-acid cation-exchange resin. The method comprises the following steps: styrene, divinylbenzen, tribromomethane, 1,4-butylene glycol and benzoyl peroxide are mixed to obtain a first preformed material; a sodium lignosulfonate is sent into a reacting furnace, a heating is carried out under the protection of nitrogen, an insulation, a cooling and a grinding are carried out, 1,3-dichloropropanol, polyalkyl silonxane and sodium hydroxide are added with stirring, hydrochloric acid is added with stirring, a filtering, a washing and a drying are carried out to obtain a second preformed material; water, polyvinyl alcohol and ethyl acrylate are mixed, methylene blue is added with stirring, a first preformed material is added with stirring, chloroform is added for swelling, a sulfuric acid solution, sulfur trioxide and a second preformed material are added with stirring, chloroform is evaporated, cooling is carried out, water is added, a standing is carried out, a water layer is removed, a washing is carried out till the pH of the solution is 7, a sodium hydroxide solution is added with stirring, a filtering is carried out, a product is washed till the pH is 7, water is added with stirring, heating, insulation and filtering are carried out, absolute ethyl alcohol is added for backflow, cooling, filtering and washing are carried out, and a drying is carried out to obtain the strong-acid cation-exchange resin.
Owner:ANHUI WANDONG CHEM
Tri[2-tri(1,3-dichloroisopropoxy)silicon acyl oxyethyl]isocyanurate compound and preparation method thereof
ActiveCN103539807APrevent secondary combustionHigh synergistic flame retardant performanceSilicon organic compoundsEpoxyAlcohol
The invention relates to a fire retardant, namely a tri[2-tri(1,3-dichloroisopropoxy)silicon acyl oxyethyl]isocyanurate compound, and a preparation method thereof; the structure of the compound is represented by a formula shown in the specification; the preparation method comprises the following steps: reacting silicon tetrachloride and equimolar 1,3-dichloro-2-propyl alcohol in an organic solvent below 25 DEG C, dropwise adding the organic solution of trihydroxyethyl isocyanurate the mol weight of which is 1 / 3 times of that of silicon tetrachloride, reacting at 80-95 DEG C for 9-13 hours, dropwise adding 1,3-dichloro-2-propyl alcohol the mol weight of which is 2-3 times of that of silicon tetrachloride, and reacting at 80-95 DEG C for 7-10 hours; adding an acid-binding agent, stirring and performing heat preservation for 1 hour; purifying to obtain the fire retardant, namely a tri[2-tri(1,3-dichloroisopropoxy)silicon acyl oxyethyl]isocyanurate compound. The compound disclosed by the invention has the advantages of being high in flame-retardant performance, simple in preparation process, low in cost and easy for industrial production and is applied to being used as the fire retardant of materials, such as polyvinyl chloride, polyurethane, epoxy resin and unsaturated resin.
Owner:SHANDONG XINGQIANG CHEM IND TECH RES INST CO LTD
Fetor pseudomonas putida ZJB09102 and application thereof in preparation of epoxy chloropropane
ActiveCN102168041AAbility of strong dehalogenation reactionSuitable for industrial productionBacteriaMicroorganism based processesEpoxyMicroorganism
The invention provides fetor pseudomonas putida ZJB09102, which can catalyze dehalogenation reaction, and application thereof in preparation of epoxy chloropropane. The strain, namely the fetor pseudomonas putida ZJB09102 is collected in China Center for Type Culture Collection the address of which is Wuhan University, Wuhan, Chain, 430072; the collection number of the strain is CCTCC No: M2010273; and the collection data of the strain is October 24, 2010. The pseudomonas putida ZJB09102 mainly has the benefits as follows: the microbial strain, namely the fetor pseudomonas putida ZJB09102, has very strong dehalogenation reaction capability; when the strain is utilized for producing the epoxy chloropropane, the conversion rate of 1,3-dichloro-2-propanol achieves 50%-90% (that is, 1 mole of the 1,3-dichloro-2-propanol can be converted to 0.50-0.90 mole of the epoxy chloropropane); and furthermore, in the production process, a chemical catalyst is not used, therefore, the strain has the characteristics of being green and environment-friendly and is applicable to the industrialized production of the epoxy chloropropane.
Owner:ZHEJIANG UNIV OF TECH
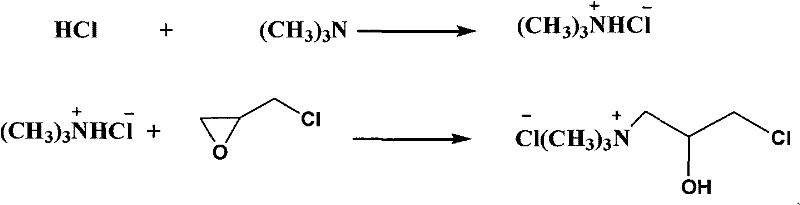
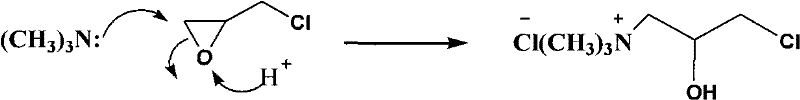
Synthesis method of epoxypropyltrimethylammonium chloride
The invention relates to a synthesis method of epoxypropyltrimethylammonium chloride. The method comprises the following steps: adding dichloroethane and epichlorohydrin in a reaction bottle in turn at 20-30 DEG C, injecting trimethylamine gas in the reaction bottle in a constant speed, reacting for 8-10h after injecting, reducing temperature to 5-15 DEG C while stopping stirring, filtrating to obtain white crystals; adding water to separate dichloroethane, heating with water bath to 60-70 DEG C, performing reduced pressure distillation, taking samples to test, stopping distillation when the concentration of epoxypropyltrimethylammonium chloride solution reaches 69%, and reducing temperature to obtain the desired product. The method overcomes the defect that the existing synthesized epoxypropyltrimethylammonium chloride contains impurities; intermediate 3-chloro-2-hydroxypropyltrimethyl ammonium chloride is not needed to prepare and the product is directly prepared so as to avoid the preparation of impurity 1,3-dichloro-2-propanol and bisquaternary ammonium salt, has high product quality and save the cost.
Owner:莫智龙
Method of preparing dichloropropanols from glycerine
ActiveUS20070167659A1High of starting materialImprove production yieldOrganic compound preparationPreparation by halogen introduction1-PropanolReaction temperature
A method of highly selective catalytic hydrochlorination of glycerine and / or monochloropropanediols to the dichloropropanol products 1,3-dichloro-2-propanol and 2,3-dichloro-1-propanol, carried out in at least one continuous reaction zone at reaction temperatures in the range of 70-140° C. and with continuous removing of the water of reaction, the liquid feed containing at least 50% by weight of glycerine and / or monochloropropanediols. The method can be carried out in a continuously operating one-step circulation reactor or a cascade of continuous flow reactors of the liquid-gas type.
Owner:SPOLEK PRO CHEMICKOU A HUTNI VYROBU
Synthesis method of 3-chloro-2-hydroxypropyl-trimethyl ammonium chloride based on solid base catalysis system
InactiveCN102452945AEasy to controlHigh purityOrganic compound preparationChemical recyclingEpoxyDistillation
The invention relates to a synthesis method of 3-cholro-2-hydroxypropyl-trimethyl ammonium chloride based on a solid base catalysis system. In the synthesis method provided by the invention, a catalyst ZnO, or a composite catalyst of ZnO and KF, or a composite catalyst of ZnO and K2CO3 or a ZnO / K2CO3 / NaOH composite catalyst is adopted; advantages of a composite solid base and a low-temperature (0-15 DEG C) and room-temperature (18-25 DEG C) two-stage reaction are combined, and trimethylamine hydrochloride, epoxy chloropropane and water are directly taken as raw materials; and the synthesis method provided by the invention has the advantages of mild reaction condition, easiness in control, less side reaction and high product purity. Continuous steam stripping and reduced-pressure distillation are adopted in refining of products, operation is simplified, and energy consumption is reduced. Liquid-phase 3-cholro-2-hydroxypropyl-trimethyl ammonium chloride can be directly taken as a raw material and subjected to one-step spray drying to obtain a solid product with the moisture content of being lower than 5%, ECH (epoxy chloropropane) content being less than or equal to 5ppm detected by gas chromatography (GC), and DCP(1,3-dichloro-2-propanol) content being less than or equal to 10ppm detected by GC; and operation is easy, convenient and efficient, and no organic volatile solvent is used.
Owner:JIANGXI CHUAN SHENG TECH CO LTD
Chemical synthesis method for OPO structured lipid
ActiveCN107473963AHigh yieldHigh purityPreparation by ester-hydroxy reactionOrganic compound preparationChemical synthesisTG - Triglyceride
The invention discloses a chemical synthesis method for an OPO (1,3-dioleic acid-2-palmitic acid triglyceride) structured lipid. The chemical synthesis method is characterized by comprising the following steps: (a) in the presence of an alkali catalyst, performing heating reflux reaction reduced pressure distillation on 1,3-dichloro-2-propanol and hexadecanoic acid methyl ester in an organic solvent, so as to obtain 1,3-dichloro-2-hexadecanoic acid ester; and (b) further putting sodium oleate into the 1,3-dichloro-2-hexadecanoic acid ester obtained in the former step, introducing nitrogen for protection, putting into a reactor with a backflow condensation device, performing a reaction for 2-5 hours at 50-100 DEG C, performing weak alkali water washing, adjusting the pH value to be neutral, drying, and performing molecular distillation or vacuum distillation, thereby obtaining high-purity OPO. The chemical synthesis method is simple and convenient in synthesis route, simple in after treatment and high in total yield (greater than 75%), and OPO can be synthesized effectively.
Owner:WUHAN POLYTECHNIC UNIVERSITY
Method for purifying cationic etherifying agent via phosphate ester extraction agent
InactiveCN103936600AHigh boiling pointReduce solubilityOrganic compound preparationAmino-hyroxy compound preparationEpoxyPhosphate
The invention discloses a method for purifying a cationic etherifying agent via a phosphate ester extraction agent. According to the method, the crude product of the cationic etherifying agent is extracted and purified by taking a phosphate ester compound as an extraction agent so as to remove epoxy chloropropane and 1,3-dichloro-2-propanol remaining in the cationic etherifying agent product. When the extraction is carried out, the final extraction agent rich in the epoxy chloropropane and the 1,3-dichloro-2-propanol is regenerated, wherein a regeneration environment is maintained as an alkaline environment by adopting an alkaline liquor; the regenerated extraction agent is recycled aiming at the extraction of the crude product of the cationic etherifying agent; and meanwhile, the recycled epoxy chloropropane is cycled into the synthesized cationic etherifying agent to be utilized. Compared with the prior art, the method disclosed by the invention is simple in process, simple and convenient to operate, less in equipment investment, economical and environmentally friendly.
Owner:TIANJIN UNIV +1
Steric hindrance amine desulfurizing agent and preparation method thereof
InactiveCN104353326AReduce circulationImprove purification efficiencyOrganic compound preparationDispersed particle separationFiltrationDistillation
The invention belongs to the technical field of preparation of chemical reagents, and in particular relates to a preparation method for steric hindrance amine desulfurizing agent 1, 3-bi-tert-butylamine-2 propanol. 1, 3-bi-tert-butylamine-2 propanol crystals can be prepared through the following steps: adding 1, 3-dichloro-2 propanol and tert-butylamine, of which the proportion is 1: 2-4, as well as a reaction medium of which the inventory is 20-200wt% of the total inventory of the raw materials in percentage by weight into a reaction kettle, and performing self-compression reaction for 2-3 hours, and performing alkali neutralization reaction, extraction, drying, filtration, reduced pressure distillation and cooling crystallization on the reaction product to obtain the 1, 3-bi-tert-butylamine-2 propanol crystals. The desulfurizing agent synthetized by the preparation method disclosed by the invention has the advantages that the steric hindrance is large, the boiling point is high, the selectivity of a desulphurization process is greatly improved, and the usage scope is greatly expanded, so that the economic benefits, the environmental benefits and the social benefits of a gas purification technology of sulfur containing natural gas, refinery gas, liquified gas and the like are improved.
Owner:SOUTHWEST PETROLEUM UNIV
Preparation method of 2-acetylchloromethoxy-1,3-dichloropropane
InactiveCN102952017AShorten the timeQuality improvementOrganic compound preparationCarboxylic acid esters preparationDichloropropaneAcetic anhydride
The invention relates to a preparation method of 2-acetylchloromethoxy-1,3-dichloropropane, which comprises the following steps: extracting the following substances: 250kg of 1,3-dichloro-2-propanol, 60kg of polyformaldehyde, 3.68kg of sulfuric acid, 250kg of acetic anhydride and 5kg of postassium acetate; adding the 1,3-dichloro-2-propanol, polyformaldehyde and sulfuric acid into a clean and dry reaction kettle, slowly heating to 96-98 DEG C within 2 hours, and keeping the temperature for 2 hours; after finishing keeping the temperature, cooling to 10 DEG C, and dropwisely adding the acetic anhydride while keeping the temperature at 15-20 DEG C; and after finishing the dropwise addition, heating to 35-40 DEG C, keeping the temperature for 10 hours, adding postassium acetate, stirring, recovering the acetic anhydride in vacuum to 100 DEG C, cooling to 20 DEG C, filtering, sucking the filtrate into a rectifying still, and collecting the fraction in the 115 DEG C / 10mmHg state, thereby obtaining the 2-acetylchloromethoxy-1,3-dichloropropane of which the weight yield is 80% and the content is greater than or equal to 95%. The invention lowers the production cost and enhances the yield.
Owner:HUBEI BAOLE PHARMA
Low-temperature quick deemulsifying agent for water-containing wax-containing erude oil and its preparation method
InactiveCN1317363CFast dehydrationGood demulsification and dehydration effectHydrocarbon oil dewatering/demulsificationEpoxyOil processing
Owner:SHANDONG UNIV
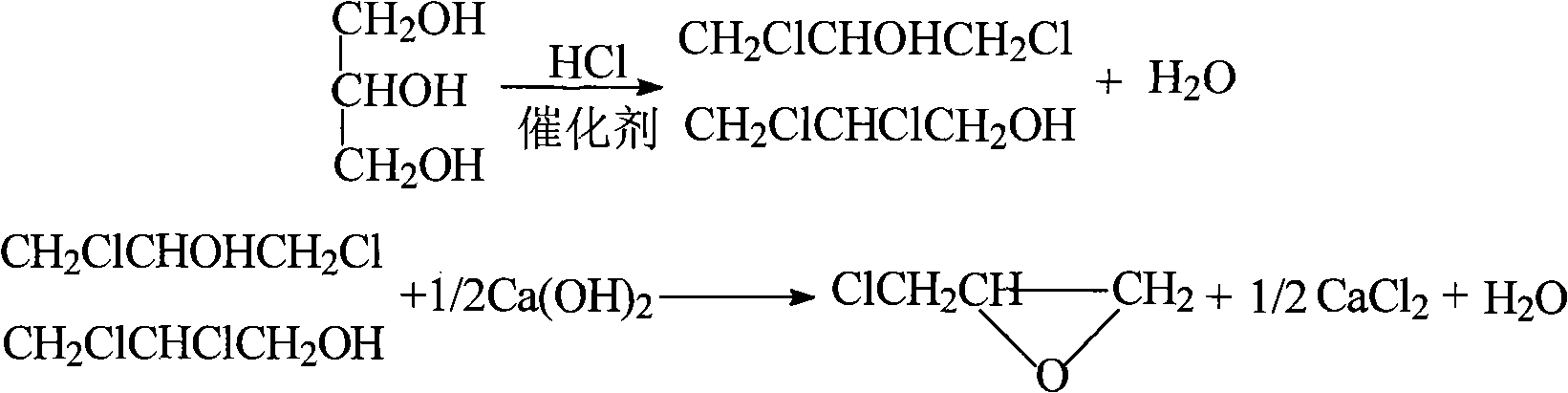
Method for directly preparing 1,3-dichloro-2-propanol by catalyzing glycerol with sulfur and zirconium
InactiveCN101613254AReduce pre-reaction timeReduce usagePhysical/chemical process catalystsPreparation by halogen introductionSulfurGlycerol
The invention relates to a method for directly preparing 1,3-dichloro-2-propanol by catalyzing glycerol with sulfur and zirconium, which comprises the following steps: using the glycerol and hydrochloric acid as raw materials according to a mol ratio of 1.0:1.0-5.0, and using the sulfur and the zirconium as catalysts; adding the glycerol and the catalysts into a reactor first; dropping the hydrochloric acid of which the mass percentage is between 20 and 38 percent into the reactor within 0.5 to 1.0 hour after starting chlorination reaction; and carrying off the water generated in the reaction process by a fractionating column of a filling glass ring to prepare the 1,3-dichloro-2-propanol, wherein the selections of the water division and the reaction condition in the reaction process are key factors for controlling the chlorination rate, and the temperature of the reaction system is adjusted and controlled by a temperature controller. By using the method for directly preparing the 1,3-dichloro-2-propanol by catalyzing the glycerol with the sulfur and the zirconium, the yield of the 1,3-dichloro-2-propanol can reach over 90 percent, the reaction condition is mild, and by using the hydrochloric acid to react with the glycerol, the post-treatment is simple and the catalysts can be recycled.
Owner:SOUTHEAST UNIV
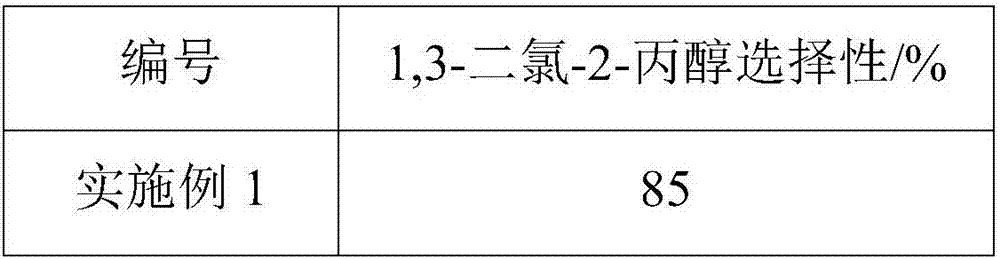
Method for preparing 1,3-dichloro-2-propanol
ActiveCN107954838AImprove responseEasy to operatePreparation by OH and halogen introductionAlcoholCarboxylic acid
The present invention discloses a method for preparing 1,3-dichloro-2-propanol. The method comprises the following steps: performing a contact reaction on allyl chloride, hydrogen chloride, an oxidantand an oxygen-containing hydrocarbon in the presence of a catalyst, wherein the oxygen-containing hydrocarbon is an alcohol and / or a carboxylic acid, and the catalyst is a titanium silicate molecularsieve catalyst. The method has the advantages of no use of toxic chlorine, simple operation process, mild reaction conditions, high selectivity of 1,3-dichloro-2-propanol, and easy separation of thecatalyst.
Owner:CHINA PETROLEUM & CHEM CORP +1
Synthesis method of epoxypropyltrimethylammonium chloride
The invention relates to a synthesis method of epoxypropyltrimethylammonium chloride. The method comprises the following steps: adding dichloroethane and epichlorohydrin in a reaction bottle in turn at 20-30 DEG C, injecting trimethylamine gas in the reaction bottle in a constant speed, reacting for 8-10h after injecting, reducing temperature to 5-15 DEG C while stopping stirring, filtrating to obtain white crystals; adding water to separate dichloroethane, heating with water bath to 60-70 DEG C, performing reduced pressure distillation, taking samples to test, stopping distillation when the concentration of epoxypropyltrimethylammonium chloride solution reaches 69%, and reducing temperature to obtain the desired product. The method overcomes the defect that the existing synthesized epoxypropyltrimethylammonium chloride contains impurities; intermediate 3-chloro-2-hydroxypropyltrimethyl ammonium chloride is not needed to prepare and the product is directly prepared so as to avoid the preparation of impurity 1,3-dichloro-2-propanol and bisquaternary ammonium salt, has high product quality and save the cost.
Owner:莫智龙
Method for Purifying Cationic Etherifying Agent with Phosphate Ester Extractant
InactiveCN103936600BHigh boiling pointReduce solubilityOrganic compound preparationAmino-hyroxy compound preparationEpoxyPhosphate
The invention discloses a method for purifying a cationic etherifying agent via a phosphate ester extraction agent. According to the method, the crude product of the cationic etherifying agent is extracted and purified by taking a phosphate ester compound as an extraction agent so as to remove epoxy chloropropane and 1,3-dichloro-2-propanol remaining in the cationic etherifying agent product. When the extraction is carried out, the final extraction agent rich in the epoxy chloropropane and the 1,3-dichloro-2-propanol is regenerated, wherein a regeneration environment is maintained as an alkaline environment by adopting an alkaline liquor; the regenerated extraction agent is recycled aiming at the extraction of the crude product of the cationic etherifying agent; and meanwhile, the recycled epoxy chloropropane is cycled into the synthesized cationic etherifying agent to be utilized. Compared with the prior art, the method disclosed by the invention is simple in process, simple and convenient to operate, less in equipment investment, economical and environmentally friendly.
Owner:TIANJIN UNIV +1
Flame retardant bis[tris(1,3-dichloro-2-propoxy)silyloxy]ethane compound and preparation method thereof
ActiveCN103554160BPrevent secondary combustionHigh synergistic flame retardant performanceSilicon organic compoundsEpoxyOrganic solvent
The invention relates to a flame retardant bis[tris(1,3-dichloro-2-propoxy)silicon-acyloxy]ethane compound and a preparation method thereof. The structure of the compound is represented by a formula shown in a drawing. The preparation method comprises the steps of reacting silicon tetrachloride with 1,3-dichloro-2-propanol of a mole which is equal to that of silicon tetrachloride in an organic solvent at the temperature below 25 DEG C, then, dropwise adding glycol of a mole which is 0.5 times that of silicon tetrachloride, heating to the temperature of 65-80 DEG C after completing dripping, and reacting for 7-10 hours; then, dropwise adding 1,3-dichloro-2-propanol of a mole which is 2-3 times that of silicon tetrachloride, and carrying out heat-preservation reaction for 6-10 hours at the temperature of 70-90 DEG C; then, adding an acid binding agent, and carrying out heat preservation for 1 hour while stirring; purifying, thereby obtaining the flame retardant bis[tris(1,3-dichloro-2-propoxy)silicon-acyloxy]ethane. The compound disclosed by the invention has high flame retarding efficacy due to the synergism of silicon and chlorine and is suitable for serving as a flame retardant for materials, such as polyvinyl chloride, polyurethane, epoxy resin, unsaturated resin and the like, and the preparation method is simple and is low in cost, so that the industrial production is facilitated.
Owner:山东产研中科高端化工产业技术研究院有限公司
A kind of gel polymer binder for lithium-sulfur battery electrode material and preparation method thereof
ActiveCN104319404BEasy to operateImprove adhesionElectrode manufacturing processesPolyethylene glycolOxygen
The invention discloses a gel polymer binder for a lithium sulfur battery electrode material and a preparation method thereof. The preparation method comprises the following steps: (1) weighing 1,3-dichloro-2-propanol and polyethylene glycol, uniformly mixing, introducing N2 to deoxygenize, adjusting temperature, keeping constant temperature, adding a catalyst to accelerate a polymerization reaction, extracting a target product 1,3-dichloro-2-propyl alcohol polyethylene glycol ether, and then adjusting the pH value of the solution with an alkaline solution; (2) weighing a sulfosalt, completely dissolving the sulfosalt in distilled water, adjusting temperature, keeping constant temperature, and adding elemental sulfur to prepare a polysulfide salt; (3) mixing a product 1,3-dichloro-2-propyl alcohol polyethylene glycol ether in the step (1) and a product polysulfide salt in the step (2), adjusting temperature, keeping constant temperature, taking out a gel-like substance, and rotatably evaporating at a certain temperature to obtain a gel polymer binder for a lithium sulfur battery. The gel polymer binder prepared by the invention has the characteristics of easiness in operation, good bonding effect, high electrode active material utilization rate, good battery rate capability and the like.
Owner:CHANGZHOU UNIV
Method for measuring contents of harmful organic chloride DCP (1,3-dichloro-2-propanol) of PAE (polyamide epichiorobydrin resin) wet strength agent in paper for daily use, and application of method
ActiveCN110470756AQuick analysisEliminate pre-processing operationsComponent separationPolyamideDerivatization
The invention relates to the technical field of detection, and discloses a method for measuring contents of the harmful organic chloride DCP (1,3-dichloro-2-propanol) of a PAE (polyamide epichiorobydrin resin) wet strength agent in paper for daily use, and is used for testing the residual quantity and the migration amount of the harmful organic chloride DCP of the PAE wet strength agent in the paper for daily use through a SPME (Solid Phase Micro Extraction)-GC-MS / MS (Gas Chromatography-Tandem Mass Spectrum). The method breaks through the bottleneck that methods for detecting the contents of the harmful organic chloride of the PAE wet strength agent in the paper for daily use are not in the presence at home and abroad. Meanwhile, compared with a method for detecting the migration amount ofthe harmful organic chloride DCP of the PAE wet strength agent with a high residual risk in paper for daily use in the European Union, the method omits a pre-processing operation of derivatization, avoids test toxicity, is convenient and efficient in operation, is more suitable for the product quality control of a production process and the quick analysis of the harmful organic chloride of the PAE wet strength agent in the paper for daily use of a circulation domain and a use link.
Owner:SOUTH CHINA UNIV OF TECH
Preparation method for flame retardant
InactiveCN107827917AImprove flame retardant performanceThe preparation method is simple through the traditional preparation processSilicon organic compoundsMaterials preparationWater baths
The invention discloses a method for preparing a flame retardant, which belongs to the field of material preparation. Include the following steps: dioxane and silicon tetrachloride in a flask; under stirring, cool with a cold water bath, add dropwise 3-dichloro-2-propanol, after the HCl gas is released, drop ethylene glycol into the four-necked flask, after the HCl gas is released, drop 3-dichloro-2-propanol, and after the HCl gas is released, add melamine, keep stirring for 1h, and the pH value of the solution reaches 5 ~ 6 as The end of the reaction: the reaction system is cooled and suction filtered, the filtrate is distilled under reduced pressure to remove dioxane and low boiling point substances, then petroleum ether is added to stir and wash, and the petroleum ether is removed under reduced pressure to obtain the product EDSD. Through the effective improvement of the traditional preparation process, the synthetic raw material of the flame retardant utilizes silicon tetrachloride, a by-product of polysilicon, and the prepared flame retardant has higher flame retardant performance, and the preparation process is simple and cost-saving. The present invention The preparation method of the flame retardant has broad application space.
Owner:SHAANXI JUJIEHAN CHEM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
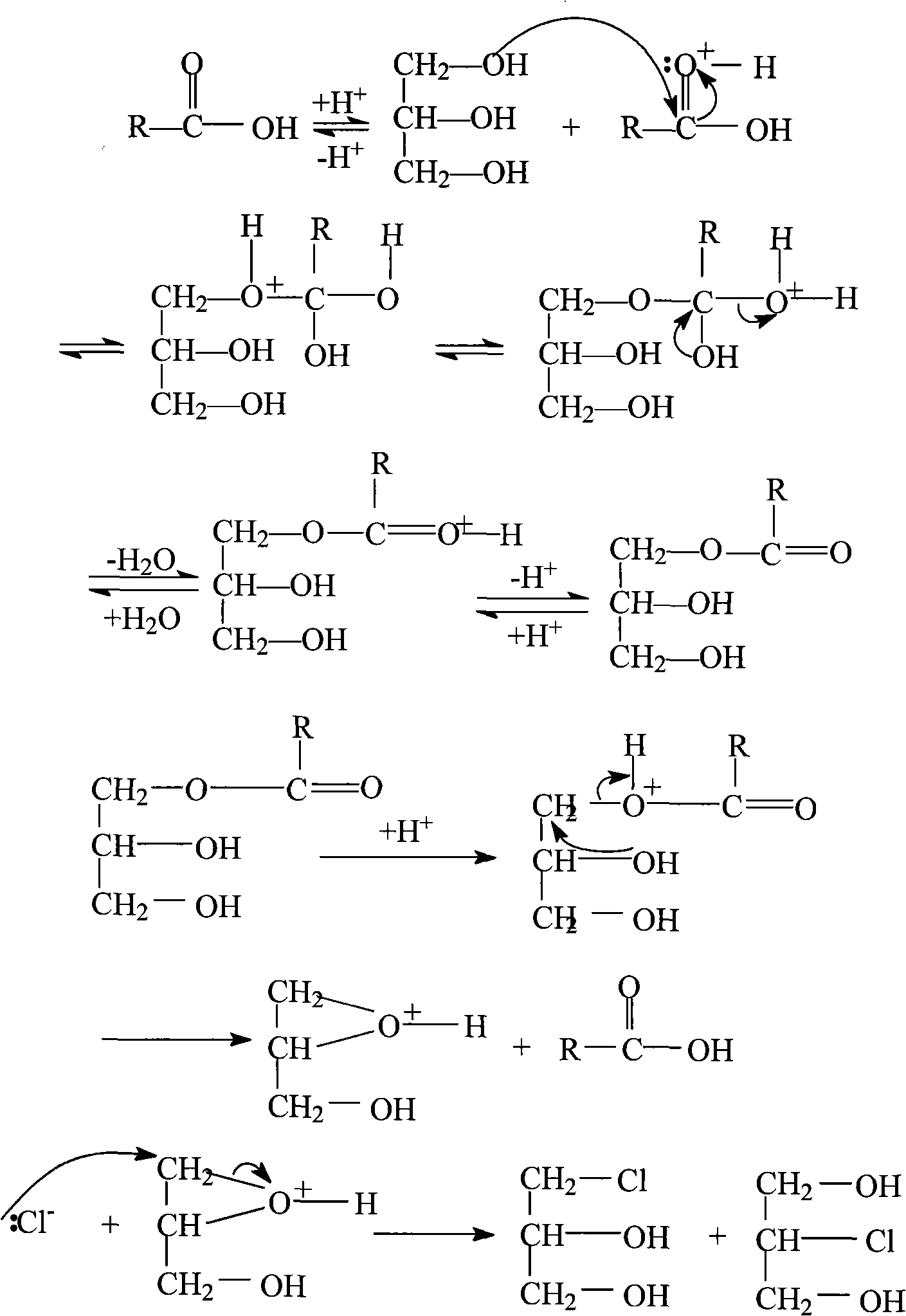
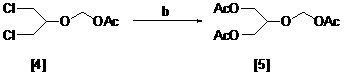
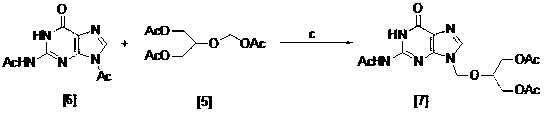
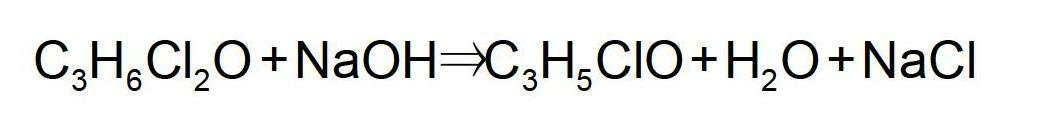
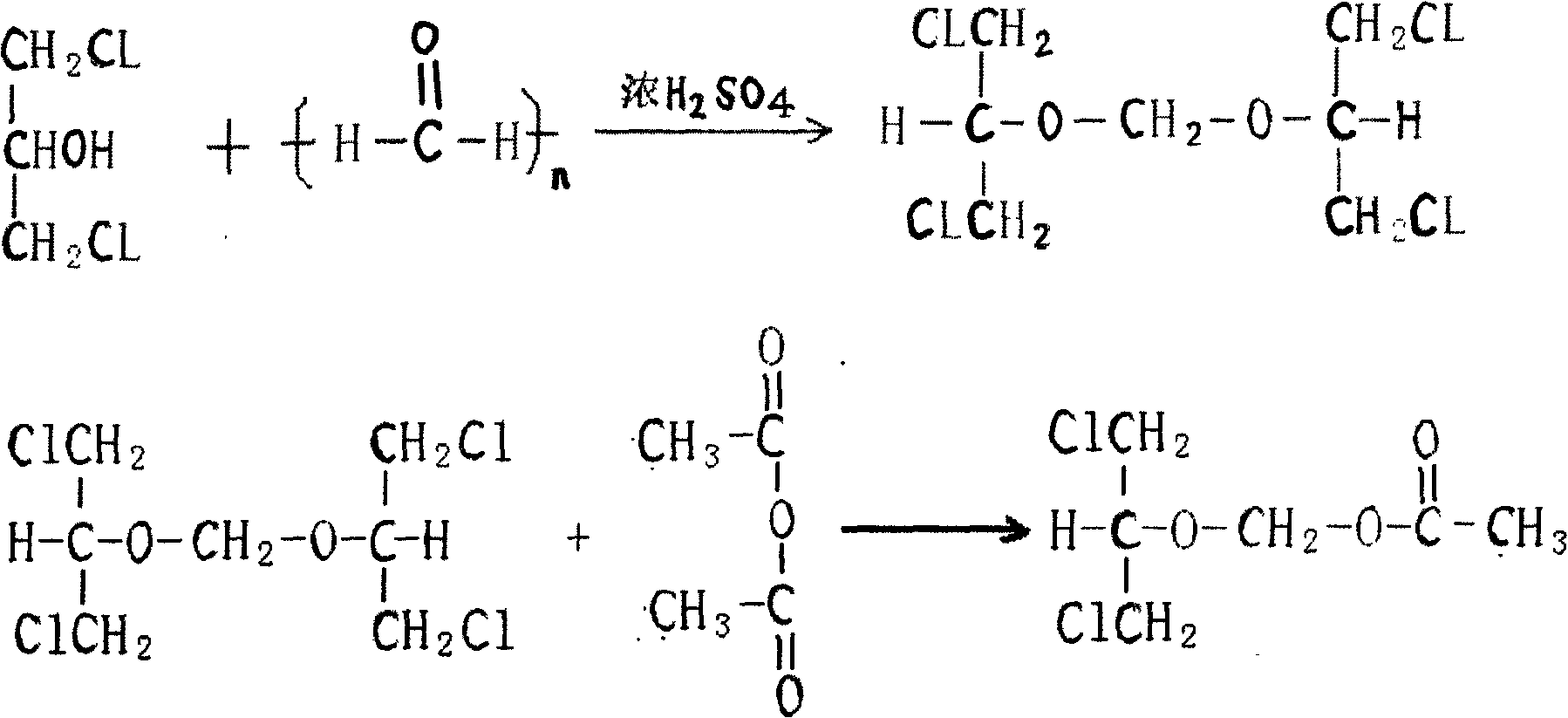

![Tri[2-tri(1,3-dichloroisopropoxy)silicon acyl oxyethyl]isocyanurate compound and preparation method thereof Tri[2-tri(1,3-dichloroisopropoxy)silicon acyl oxyethyl]isocyanurate compound and preparation method thereof](https://images-eureka.patsnap.com/patent_img/c20b4bdd-8f43-478b-8a0a-6c041cbef578/HSA0000097406390000011.PNG)
![Tri[2-tri(1,3-dichloroisopropoxy)silicon acyl oxyethyl]isocyanurate compound and preparation method thereof Tri[2-tri(1,3-dichloroisopropoxy)silicon acyl oxyethyl]isocyanurate compound and preparation method thereof](https://images-eureka.patsnap.com/patent_img/c20b4bdd-8f43-478b-8a0a-6c041cbef578/HSA0000097406390000012.PNG)
![Tri[2-tri(1,3-dichloroisopropoxy)silicon acyl oxyethyl]isocyanurate compound and preparation method thereof Tri[2-tri(1,3-dichloroisopropoxy)silicon acyl oxyethyl]isocyanurate compound and preparation method thereof](https://images-eureka.patsnap.com/patent_img/c20b4bdd-8f43-478b-8a0a-6c041cbef578/BSA0000097406380000021.PNG)

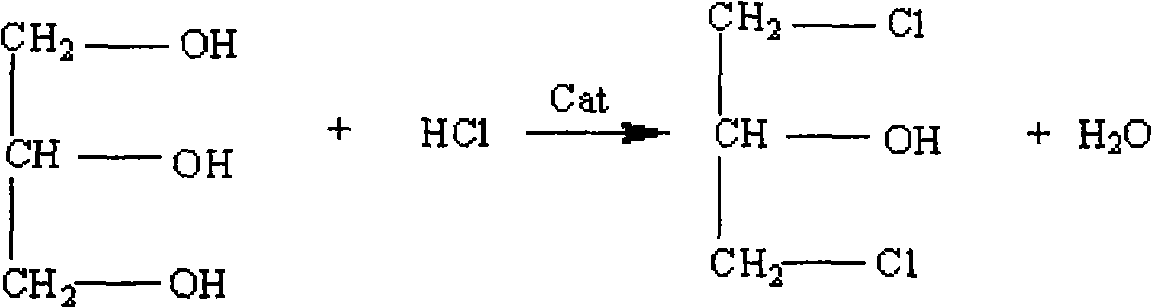
![Flame retardant bis[tris(1,3-dichloro-2-propoxy)silyloxy]ethane compound and preparation method thereof Flame retardant bis[tris(1,3-dichloro-2-propoxy)silyloxy]ethane compound and preparation method thereof](https://images-eureka.patsnap.com/patent_img/2478340c-182b-4447-90c0-7af281bc1651/HSA0000097406840000011.PNG)
![Flame retardant bis[tris(1,3-dichloro-2-propoxy)silyloxy]ethane compound and preparation method thereof Flame retardant bis[tris(1,3-dichloro-2-propoxy)silyloxy]ethane compound and preparation method thereof](https://images-eureka.patsnap.com/patent_img/2478340c-182b-4447-90c0-7af281bc1651/HSA0000097406840000012.PNG)
![Flame retardant bis[tris(1,3-dichloro-2-propoxy)silyloxy]ethane compound and preparation method thereof Flame retardant bis[tris(1,3-dichloro-2-propoxy)silyloxy]ethane compound and preparation method thereof](https://images-eureka.patsnap.com/patent_img/2478340c-182b-4447-90c0-7af281bc1651/BSA0000097406830000021.PNG)