Preparation method of 1,3-dihydroxyacetone
A technology of dihydroxyacetone and acetone, applied in the field of preparation of 1,3-dihydroxyacetone, achieving high efficiency and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The invention provides a kind of preparation method of 1,3-dihydroxyacetone, comprises the following steps:
[0023] (1) Under the condition that catalyst exists, carry out contact reaction with glycerol and halogenating agent, prepare 1,3-dichloro-2-propanol;
[0024] (2) subjecting 1,3-dichloro-2-propanol to oxidative dehydrogenation reaction to obtain intermediate product 1,3-dichloro-2-acetone;
[0025] (3) Contacting 1,3-dichloro-2-acetone with an alkaline substance in an aqueous medium for hydrolysis reaction to obtain 1,3-dihydroxyacetone, wherein the temperature of the hydrolysis reaction is 25-60°C.
[0026] According to the present invention, specifically, in step (1), the contact reaction is: mixing glycerol and catalyst in an organic solvent in proportion to form a mixed system, then adding a halogenating reagent dropwise to the mixed system, and then At 50-120°C, react for 1-10 hours.
[0027] According to the present invention, the organic solvent used i...
Embodiment 1
[0047] Mix 0.2mol glycerol and 1g catalyst in 100mL acetone to form a mixed system (the catalyst is zirconia loaded with sulfuric acid, and the quality of sulfuric acid is 8% of the quality of zirconia), and then feed the mixed system at a rate of 9mL / min. 40 mL of hydrochloric acid with a mass fraction of 35% was added dropwise to the mixture, and then reacted at 80° C. for 2 hours. After the reaction was completed, a mixed product was obtained;
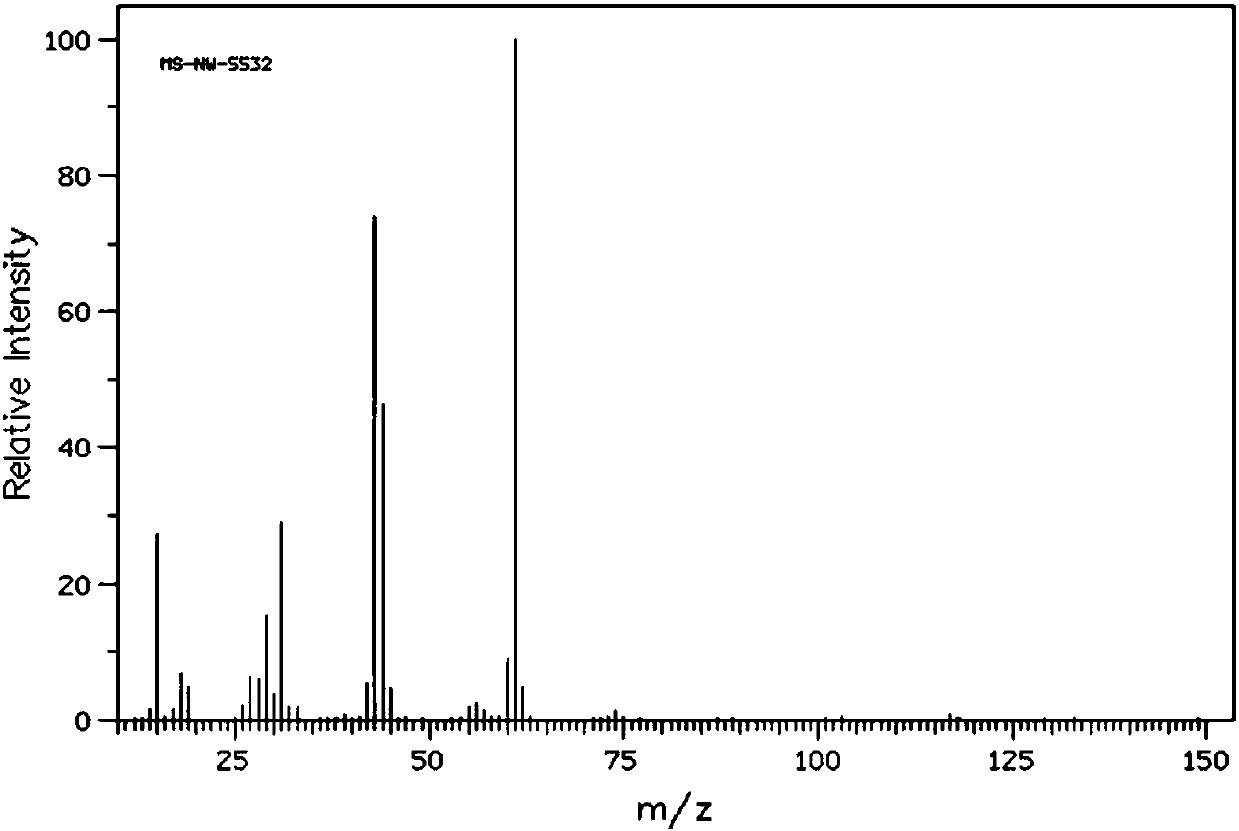
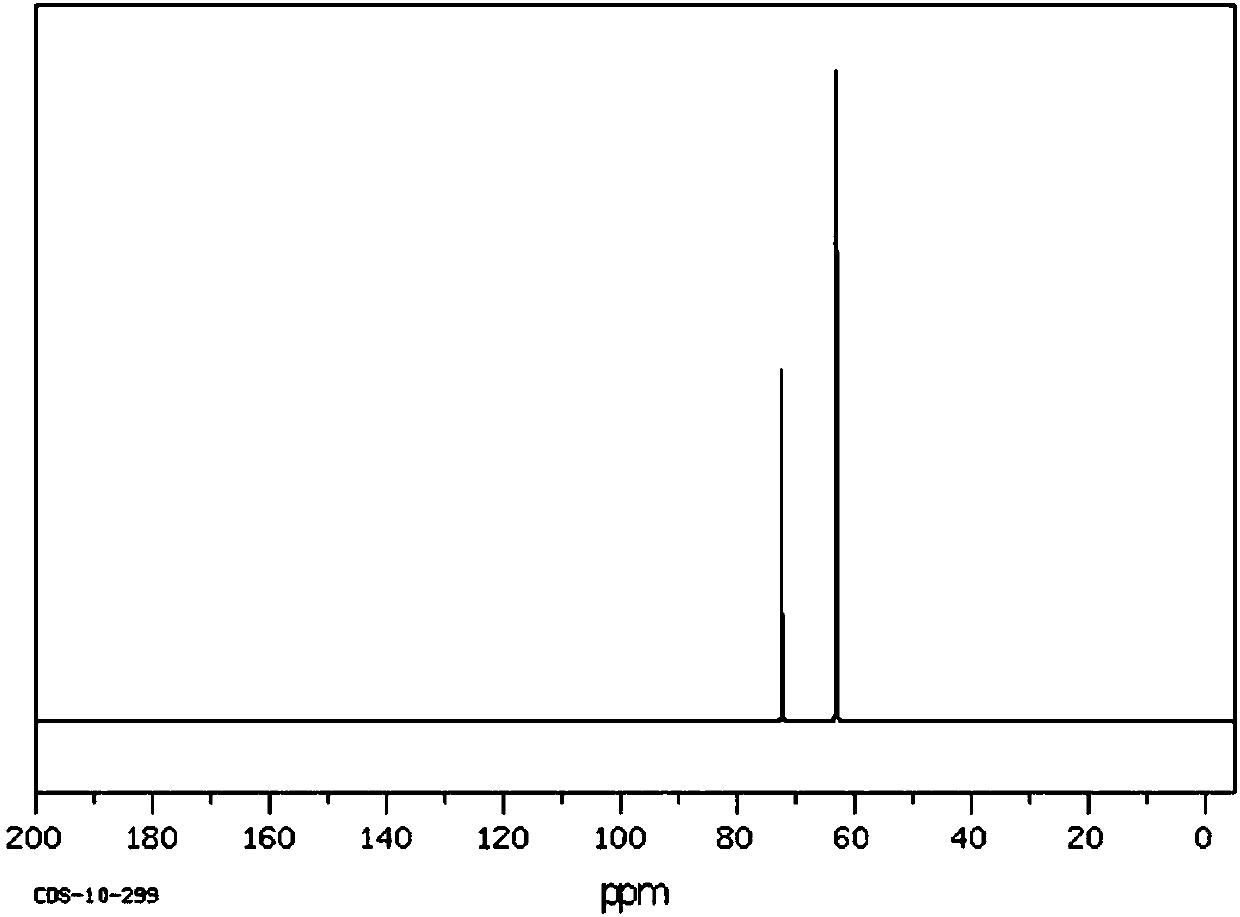
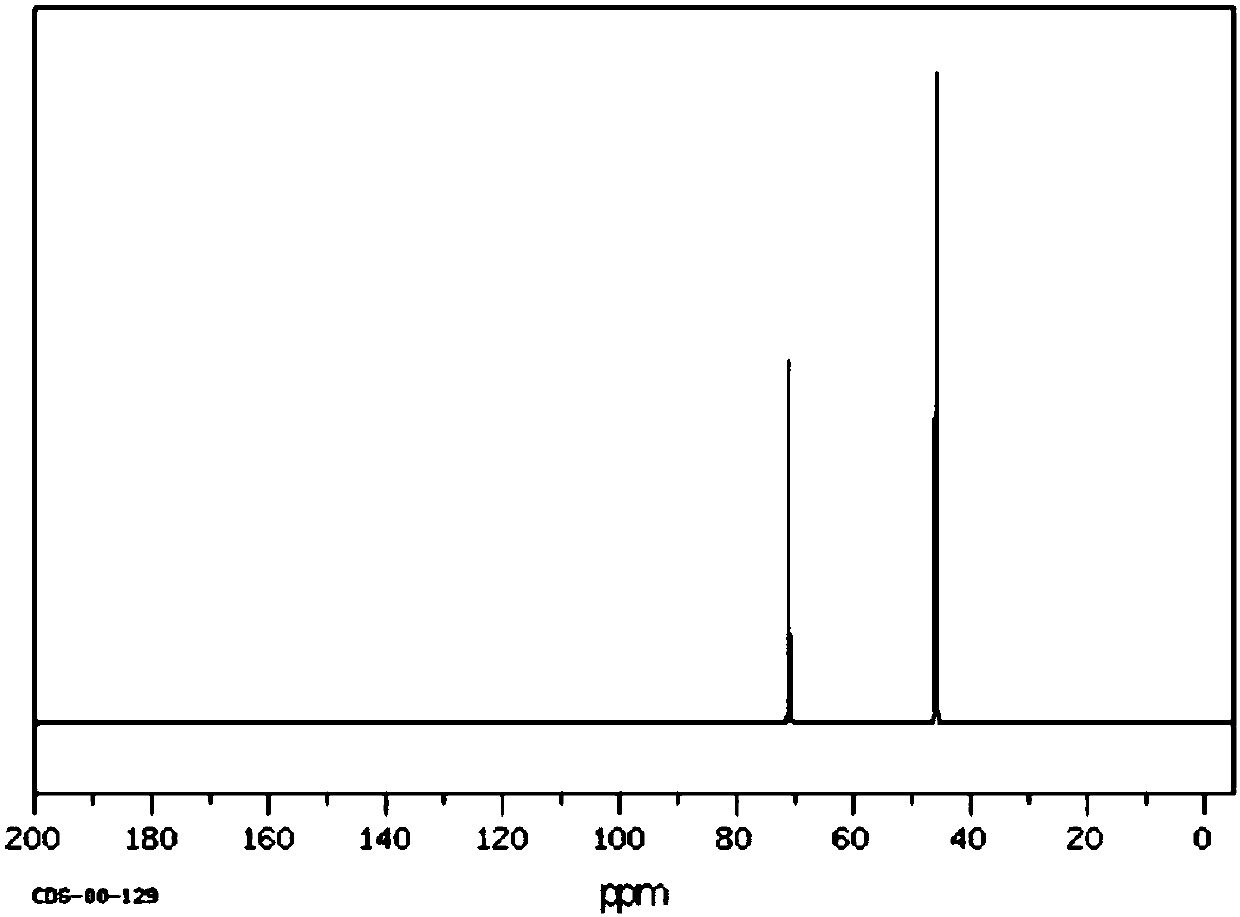
[0048] Filter the mixed product, collect the filter residue (the main component of the filter residue is the catalyst), extract the filtrate 3 times with 100 mL of water, take the lower layer solution, and carry out vacuum distillation on the lower layer solution to obtain 25.8 g (0.2 mol) of 1,3-dichloro-2 - propanol, its NMR 13 C spectrum such as image 3 As shown, its high-resolution mass spectrum is shown in Figure 4 as shown, 13 The peaks at the chemical shifts of 45.8 and 71 in the C NMR spectrum were assigned to the termi...
Embodiment 2
[0052] 0.2mol glycerol and 1.2g catalyst are mixed uniformly in 100mLN-methylpyrrolidone to form a mixed system (the catalyst is zirconia loaded with sulfuric acid, and the quality of sulfuric acid is 10% of the quality of zirconia), and then 6mL / min Add 26mL of hydrobromic acid with a mass fraction of 40% dropwise to the mixing system at a high speed, and then react at 60°C for 5h. After the reaction ends, a mixed product is obtained;
[0053] Filter the mixed product, collect the filter residue, extract the filtrate 3 times with 100 mL of water, take the lower layer solution, and carry out vacuum distillation on the lower layer solution to obtain 23.64 g (0.183 mol) of 1,3-dichloro-2-propanol;
[0054] Put 100ml of water in an ice-water bath, then add 0.18mol of 1,3-dichloro-2-propanol, 0.18mol of sodium bromide and 0.27mol of sodium dichloroisocyanurate into the water, mix thoroughly to obtain a mixed solution, and add Glycine-sodium hydroxide buffer was added to the soluti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com