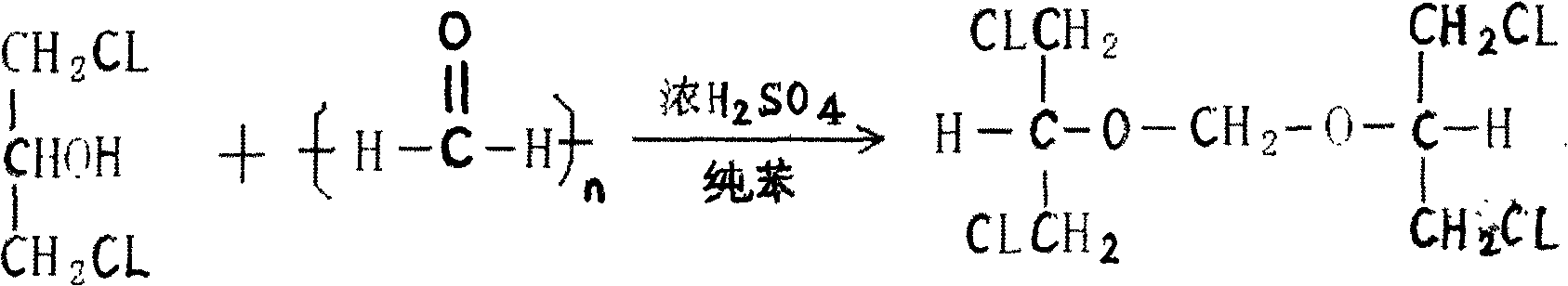
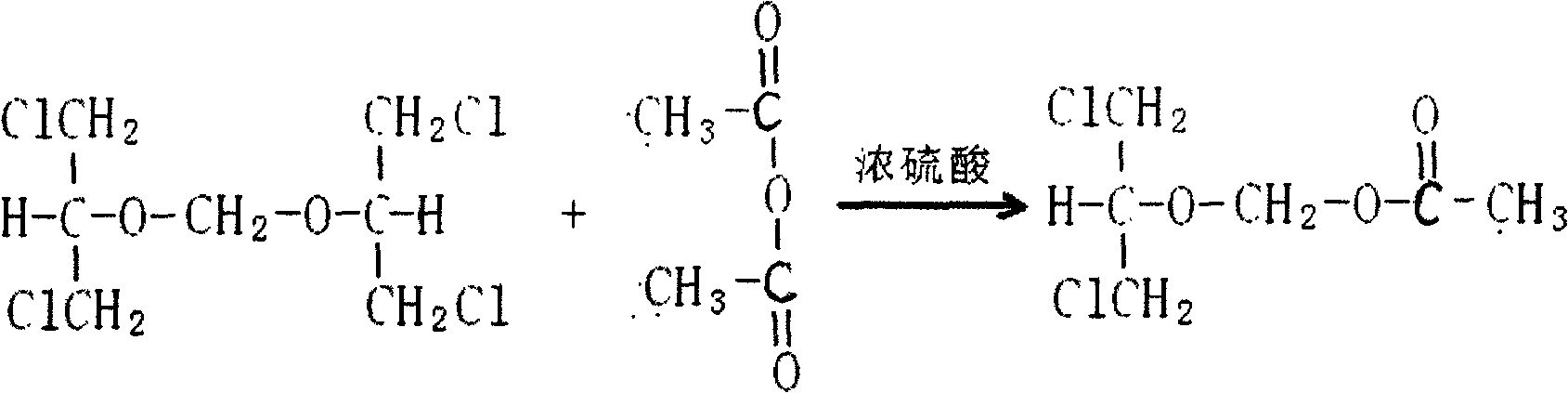
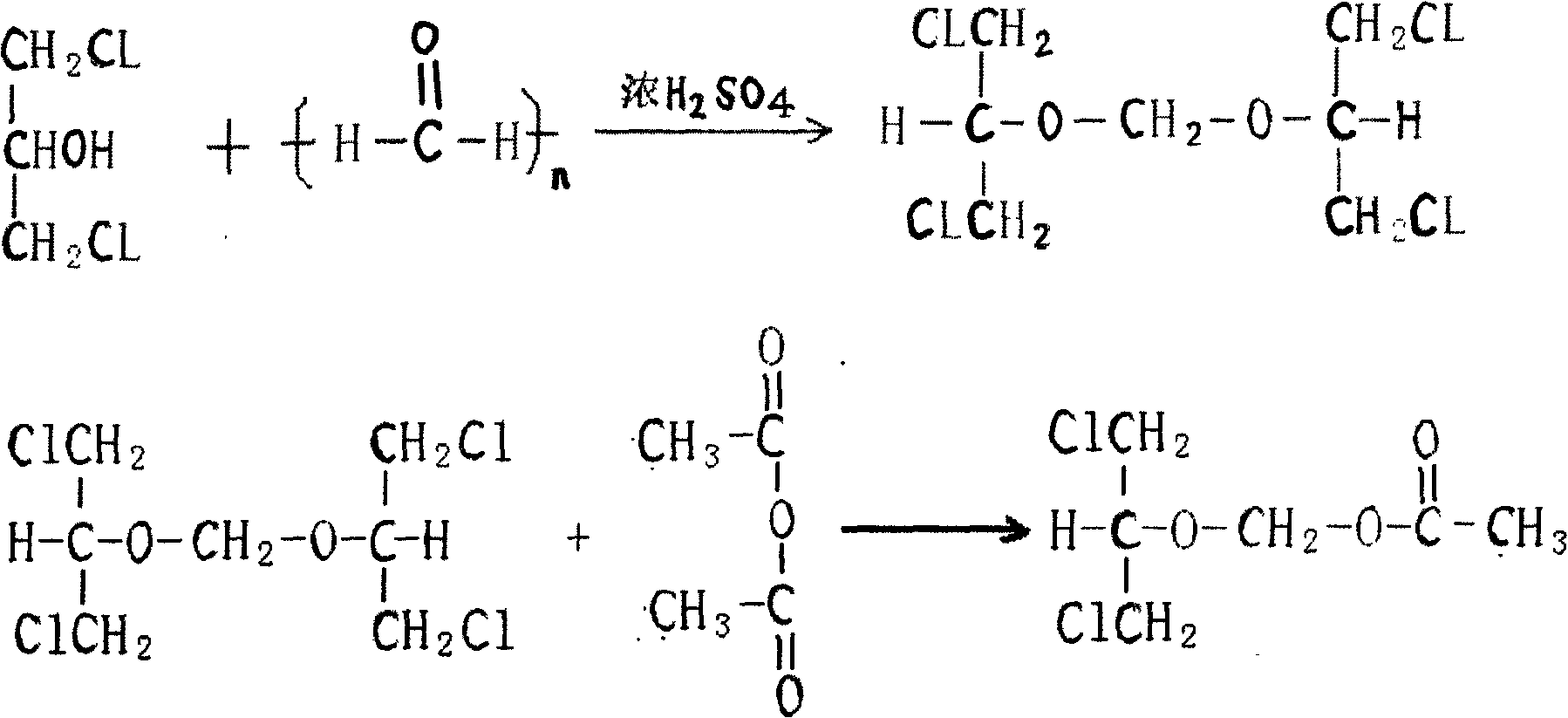Preparation method of 2-acetoxyl group methoxy group-1.3-propylene dichloride
A technology of acetoxymethoxy and dichloropropane, which is applied in the field of preparation of 2-acetoxymethoxy-1.3-dichloropropane, can solve the problems of high cost, inconvenient use, easy discoloration, etc. The effect of short time, less side reactions and less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Extract the following substances by weight ratio:
[0031]
[0032] In a clean and dry reaction kettle, add 1.3-dichloro-2-propanol, paraformaldehyde, and sulfuric acid, slowly raise the temperature, raise the temperature to 96-98°C in 2 hours, keep it warm for 2 hours, after the heat preservation is completed, cool down to 10°C and start dripping Add acetic anhydride, keep the temperature at 15-20°C, after the addition, raise the temperature at 35-40°C and keep it warm for 10 hours, add potassium acetate, stir, recover the acetic anhydride in vacuum to 100°C, cool down to 20°C, filter, and pump the filtrate into the refined In the still, collect 115°C / 10mmHg fractions to obtain 2-acetoxymethoxy-1.3-dichloropropane with a weight yield of 80% and a content of ≥95%.
Embodiment 2
[0034] Starting from the second charge, extract the following substances by weight ratio:
[0035]
[0036]
[0037] In a clean and dry reaction kettle, add 1.3-dichloro-2-propanol, paraformaldehyde, sulfuric acid, slowly raise the temperature, raise the temperature to 96-98°C in 2 hours, keep it warm for 2 hours, after the heat preservation is complete, cool it down to 10°C, add 250kg of fractions steamed below 115°C / 10mmHg during the rectification described in Example 1, then start to add acetic anhydride dropwise, the temperature remains at 15-20°C, after the addition is completed, heat up at 35-40°C for 10 hours, Add potassium acetate, stir, vacuum recover acetic anhydride to 100°C, cool down to 20°C, filter, pump the filtrate into a rectification kettle, collect 115°C / 10mmHg fractions, and obtain a weight yield of 90% and a content of ≥95%. 2-Acetoxymethoxy-1,3-dichloropropane.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com