Method for detecting volatile organic chlorides in resin by using headspace gas chromatography
A headspace gas chromatography and volatile organic technology, which is used in measuring devices, instruments, scientific instruments, etc., can solve the problems of chromatographic column pollution, detection errors, cumbersome and time-consuming preprocessing steps, etc. The effect of good precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Sample preparation and determination
[0038] 1. Sample preparation
[0039] First, add 5mL of PAE sample and 1.5g of sodium chloride into two 20mL headspace bottles in parallel; then add 10μL of ECH (1%) and 30μL of pure DCP into the second headspace bottle quickly sealed after
[0040] 2. Determination of samples
[0041] Put the headspace sample vial in the headspace autosampler, under the given conditions, after equilibrium, detect by gas chromatography, and record the gas phase signal values of ECH and DCP.
Embodiment 2
[0043] Chromatographic separation effect of ECH and DCP
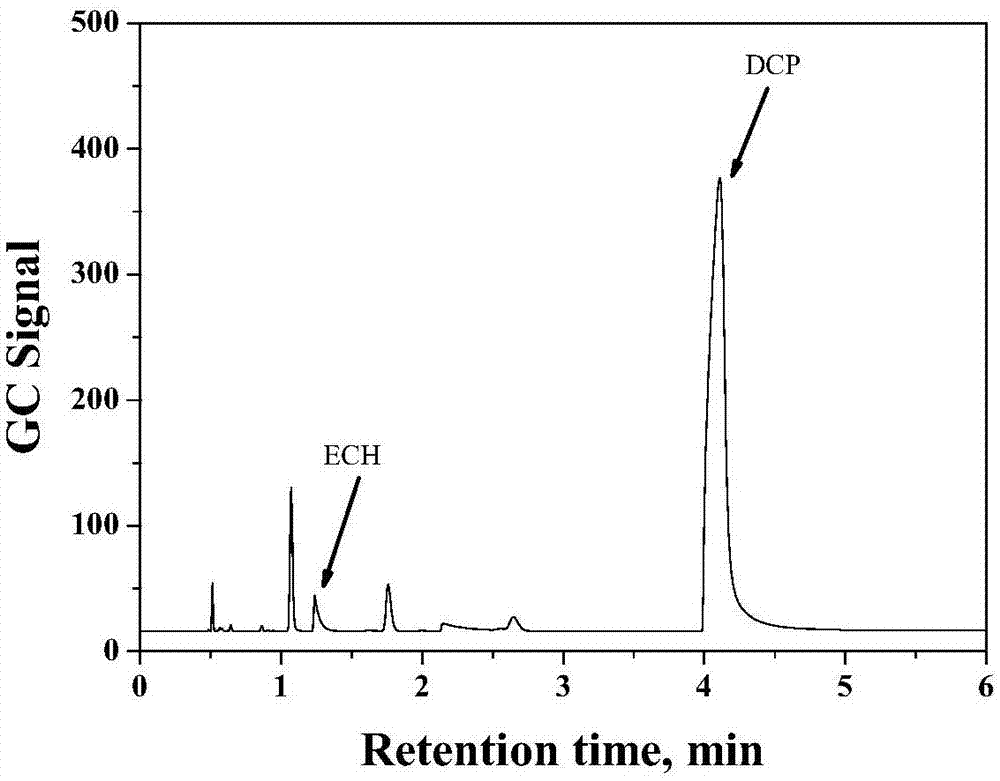
[0044] figure 1 It is the chromatographic separation peak of a typical commercially available PAE sample under the given GC conditions. It can be seen from the figure that despite the miscellaneous peaks of other volatile substances, ECH and DCP can be well separated from the polymer and will not be affected by the miscellaneous peaks. The peak times are 1.2 and 4.0 respectively about min. The separation results also showed that the gas phase signal (corresponding to the gas phase concentration) of DCP was much higher than the ECH signal in HS-GC detection.
Embodiment 3
[0046] headspace equilibrium condition
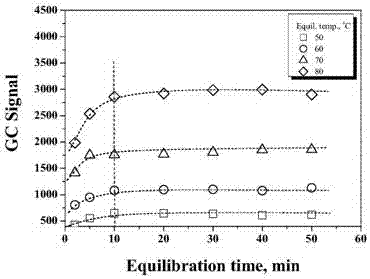
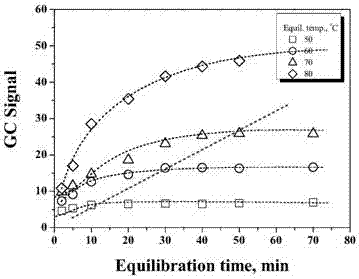
[0047] For resin solution samples, it is necessary to achieve gas-liquid phase equilibrium of the analyte before GC detection. Figure 2 is the effect of different temperatures on the equilibration time of ECH and DCP in PAE samples. It can be seen from Figure 2 that DCP can reach phase equilibrium within 10 min, however, it takes longer time for ECH to reach phase equilibrium at high temperature. This anomaly is due to the gelation of PAE, which can seriously affect the equilibration time of ECH, especially at low levels.
[0048] Relatively short phase equilibration time can improve the analytical efficiency of the instrument, therefore, this study suggests to choose the phase equilibration time at low temperature. However, as shown in Figure 2, low temperature will affect the detection sensitivity of HS-GC. Considering all the factors, 60°C and 30 min were selected as the headspace equilibrium temperature and time in the subsequent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com