Method for preparing 1,3-dichloro-2-propanol
A technology of propanol and chloropropene, which is applied in the field of preparing 1,3-dichloro-2-propanol, can solve the problems of many by-products and low selectivity of 1,3-dichloro-2-propanol, and achieve The operation process is simple, the separation energy consumption is low, and the process is safe and efficient.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
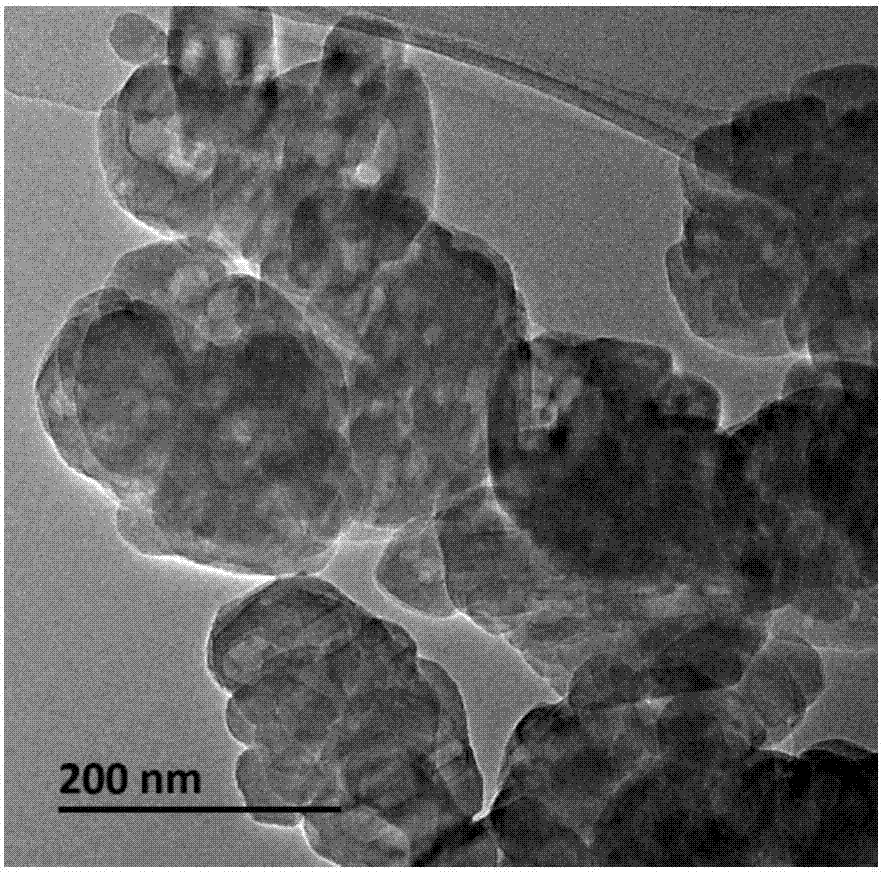
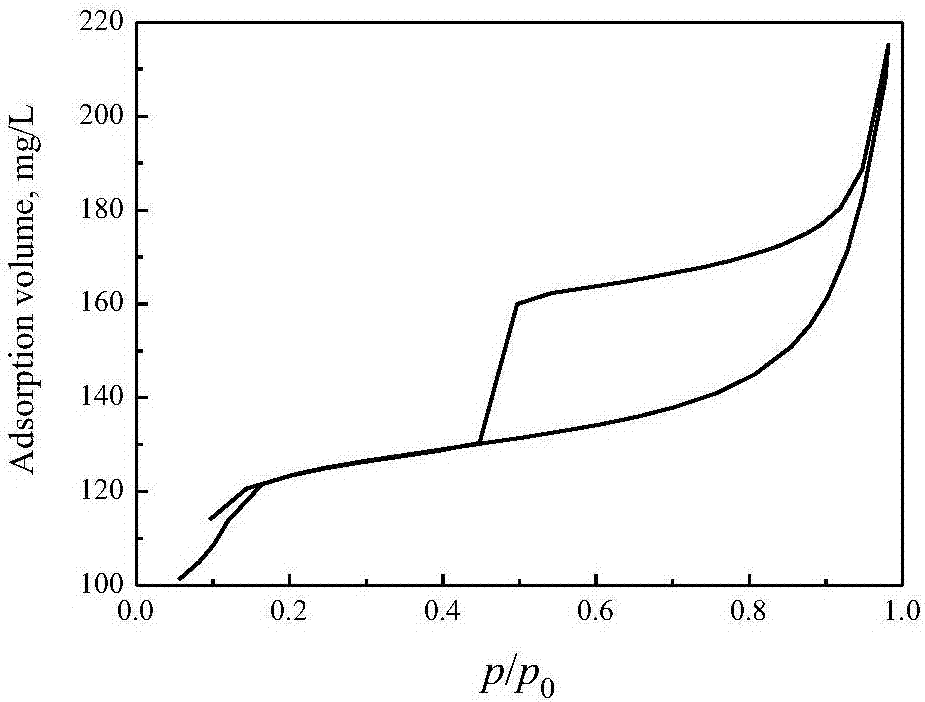
[0045] The preparation method of the TS-2 molecular sieve adopted in the embodiment is: a certain amount of tetrabutylammonium hydroxide solution (TBAOH, 20%) is mixed with tetrabutylammonium hydroxide solution (TBAOH, 20%) and tetraethyl orthosilicate (TEOS), then under the condition of violent stirring, to the obtained Add the required amount of n-butyl titanate [Ti(OBu) 4 ] of anhydrous isopropanol solution, stirred for 30 minutes to obtain a clear liquid after the completion of hydrolysis. Finally, 2 times the required amount of distilled water was added, and the resulting sol was stirred at 348-353K for 2 h to remove alcohol. The chemical composition of the obtained sol is 0.20TBAOH: SiO 2 : 0.03TiO 2 : 20H 2 O. The sol was crystallized at 443K for 3 days, and the obtained crystallized product was filtered, washed with water, dried at 373K for 6 hours, and then calcined at 823K for 16 hours to obtain a molecular sieve sample. Wherein, the consumption of TEOS is 42g, ...
Embodiment 1
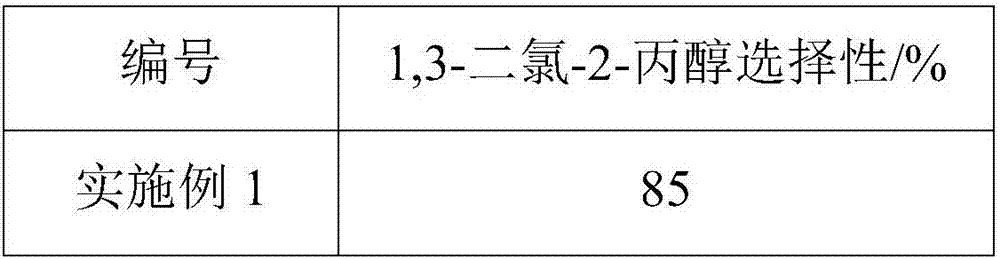
[0052] Put chloropropene, 30 mass % hydrogen peroxide aqueous solution, 37 mass % hydrochloric acid, ethanol and the required amount of water into the reaction kettle to obtain chloropropene, hydrogen peroxide, hydrogen chloride, ethanol and water in a molar ratio of 1: 1.5: 1.5: 0.1: 30 reaction mixture, put TS-1 molecular sieve into the reaction kettle, the mass ratio of TS-1 molecular sieve to chloropropene is 0.28, and then make the reaction mixture in the reaction kettle react at a temperature of 30°C 5h, after the reaction was completed, samples were taken for analysis, and the analysis results are shown in Table 1.
Embodiment 2
[0054] Put chloropropene, 30 mass % hydrogen peroxide aqueous solution, 37 mass % hydrochloric acid, trifluoroethanol and required amount of water into the reaction kettle to obtain chloropropene, hydrogen peroxide, hydrogen chloride, trifluoroethanol and water moles Ratio is 1: 1.5: 1.5: 0.1: 30 reaction mixture, put TS-1 molecular sieve into this reactor, the mass ratio of TS-1 molecular sieve and chloropropene is 0.28, then make the reaction mixture in the reactor at 30 ℃ Under the temperature of reaction 3h, take a sample analysis after completion of reaction, analysis result is shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com