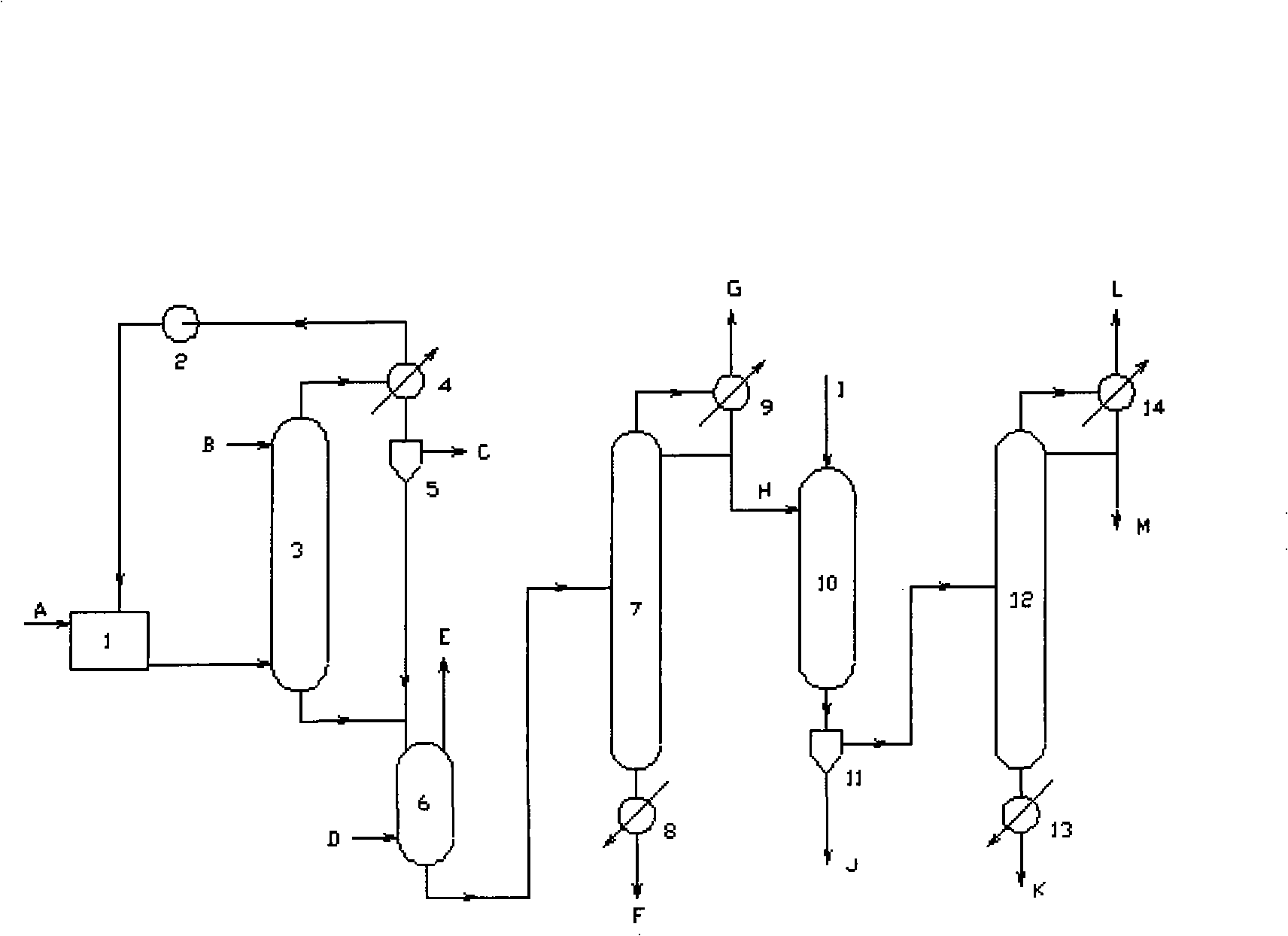
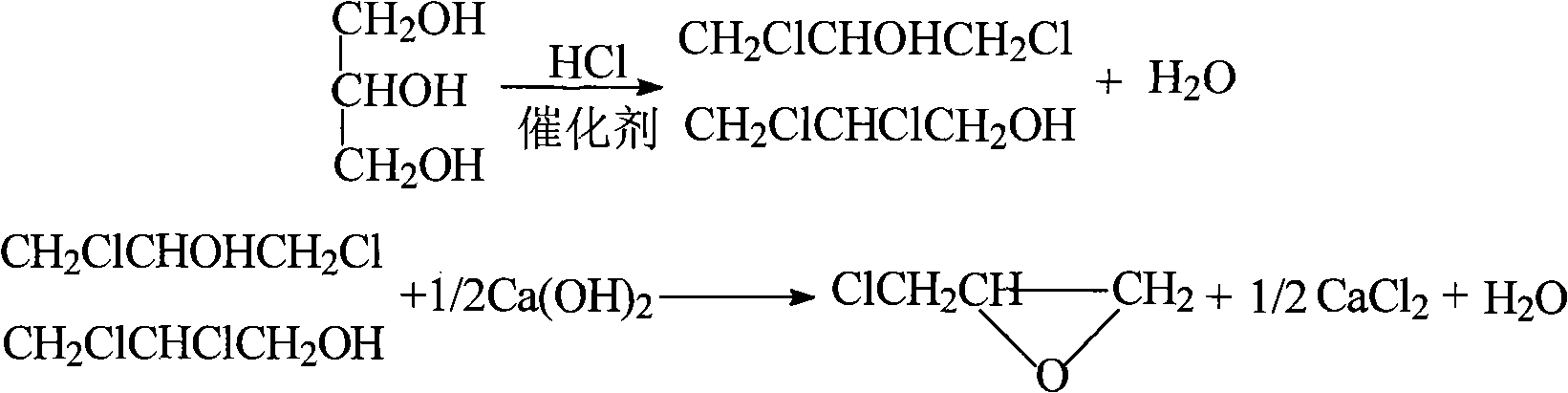
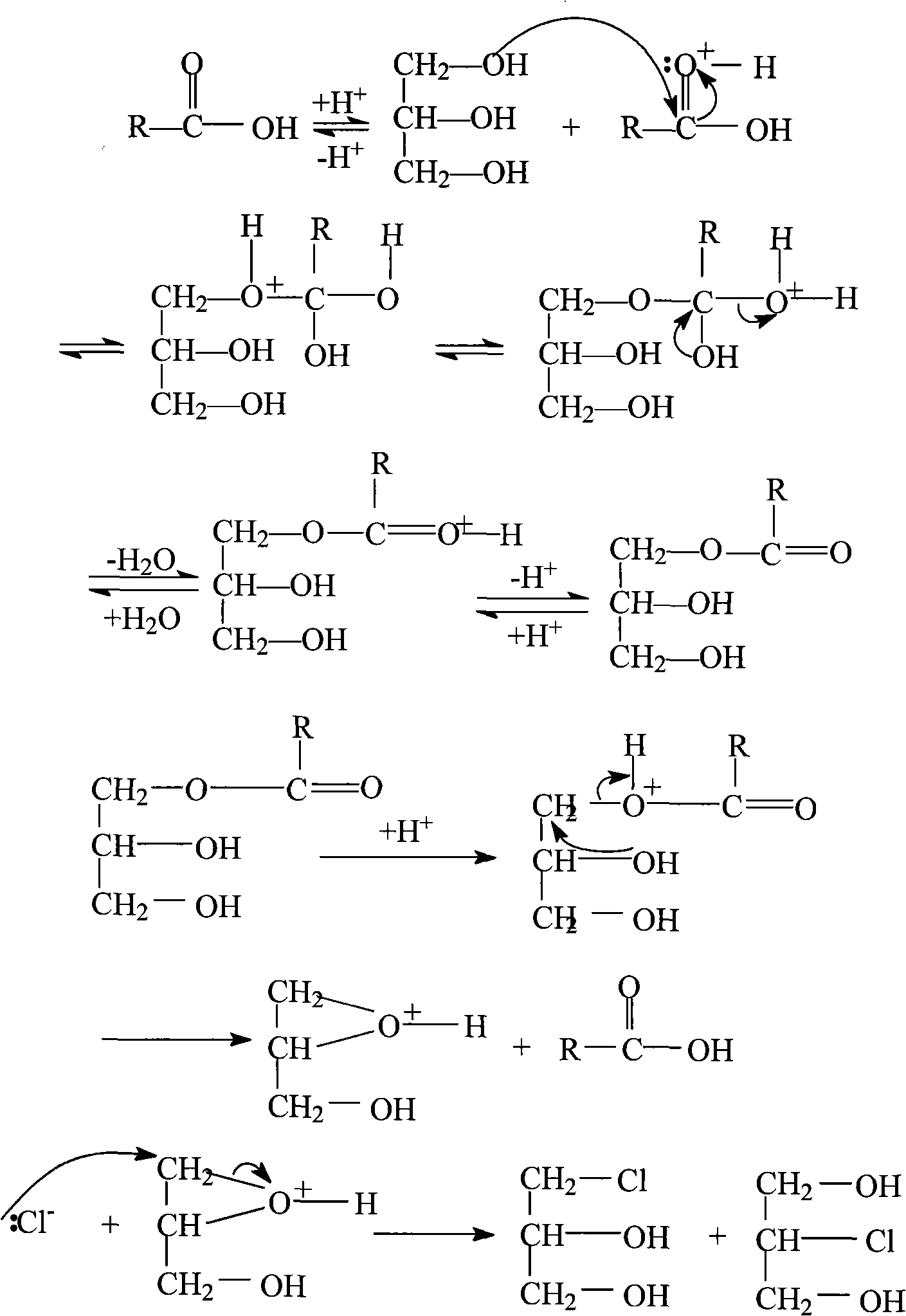Method for continuously preparing epichlorohydrin by glycerine reaction fractional distillation
An epichlorohydrin, reactive distillation technology, applied in chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, organic chemistry, etc. The effect of mild reaction conditions and high water content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] 300g glycerol, 3g oxalic acid and 1.5g ZnCl 2 , 0.75gFeCl 2 , 0.75g CuCl, 0.3g benzoyl peroxide, and 0.3g hydroquinone are added intermittently or continuously from the top of the chlorination reaction tower, and 450g of dried hydrogen chloride is introduced from the bottom of the tower to control the reaction temperature at 105°C. The chlorinated mixed liquid is sent to the degassing tower to release hydrogen chloride, rectified under reduced pressure, and tracked and detected by gas chromatography. The fraction collected at 68~75°C (1.87KPa) is 1,3-dichloro-2-propanol and 2,3 -The mixture of dichloro-1-propanol, and then carry out saponification and cyclization with 450g NaOH solution of 10% mass concentration in the saponification reaction tower to obtain 216g of epichlorohydrin product. The basic parameters and results described are shown in the table below:
[0068]
Embodiment 2
[0070] 300g glycerin, 4.5g malonic acid and 2g ZnCl 2 , 0.5gFeCl 3 , 0.5gTiCl 4, 0.45g of benzoyl peroxide, and 0.45g of hydroquinone are added intermittently or continuously from the top of the chlorination reaction tower, and 462g of dried hydrogen chloride is introduced from the bottom of the tower, and the reaction temperature is controlled at 108°C. The liquid is sent to the degassing tower to release hydrogen chloride, rectified under reduced pressure, tracked and detected by gas chromatography, and the fraction collected at 68~75°C (1.87KPa) is 1,3-dichloro-2-propanol and 2,3-dichloro - the mixture of 1-propanol, and then carry out saponification and cyclization with 430g NaOH solution of 15% mass concentration in the saponification reaction tower to obtain 220g of epichlorohydrin product. The basic parameters and results described are shown in the table below:
[0071]
Embodiment 3
[0073] 500g glycerol, 10g succinic acid and 5gZnCl 2 , 1.25gFeCl 2 , 1.25gKCl, 0.75g benzoyl peroxide, and 0.75g hydroquinone are added intermittently or continuously from the top of the chlorination reaction tower, and 450g of dried hydrogen chloride is introduced from the bottom of the tower to control the reaction temperature at 105°C. The chlorinated mixed liquid is sent to the degassing tower to release hydrogen chloride, rectified under reduced pressure, and tracked and detected by gas chromatography. The fraction collected at 68~75°C (1.87KPa) is 1,3-dichloro-2-propanol and 2,3 -The mixture of dichloro-1-propanol, then carry out saponification and cyclization with 550g NaOH solution of 20% mass concentration in the saponification reaction tower to obtain 360g of epichlorohydrin product. The basic parameters and results described are shown in the table below:
[0074]
[0075]
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com