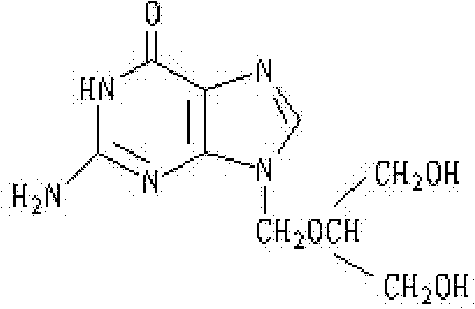
Pharmaceutical composition containing ganciclovir compound, and preparation method thereof
A technology of ganciclovir and its composition, which is applied in the field of antiviral drug preparations, can solve the problems of reduced dissolution rate of ganciclovir and failure to meet the requirements of ganciclovir injection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
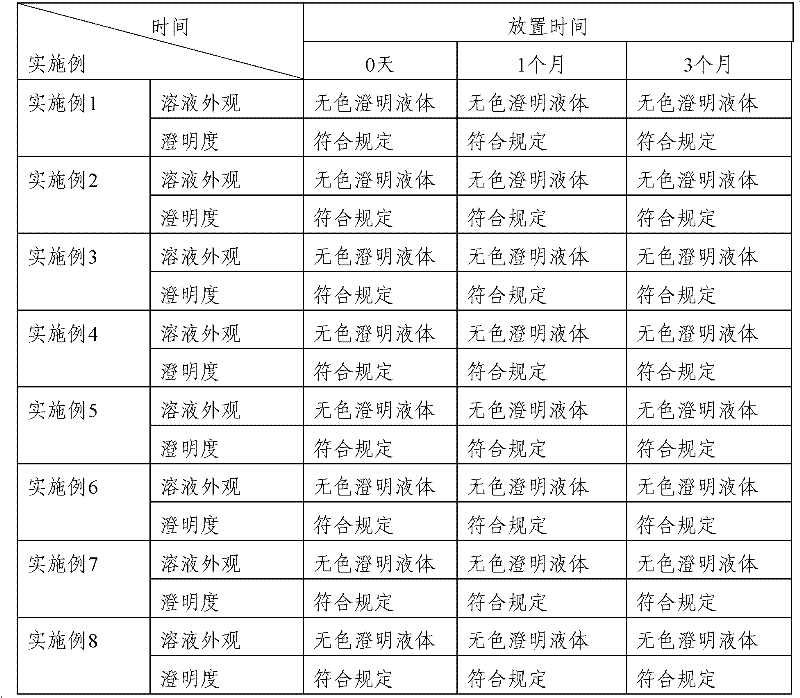
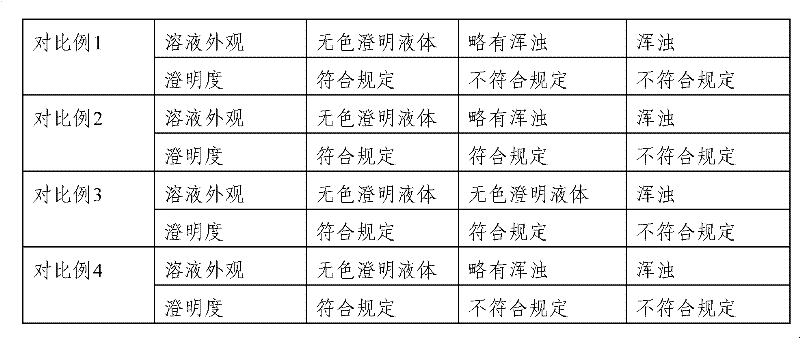
Embodiment 1
[0041] 1. Formula (weight: mg): Ganciclovir 250, Dextran 50, Sodium Hydroxide 6.5 and Polysorbate 80 60.
[0042] 2. Preparation method:
[0043] 1) Add the prescribed amount of lactose and dextran into an appropriate amount of water for injection, and stir until completely dissolved to obtain solution 1);
[0044] 2) Dissolve sodium hydroxide in an appropriate amount of water for injection, add ganciclovir and stir, and control the pH value to 10.5 to obtain solution 2);
[0045] 3) Combine solutions 1) and 2), add activated carbon with a prepared volume of 0.5%, boil for 30 minutes, filter and decarbonize, add water for injection to the full amount, and then filter to the storage tank through a sterilizing filter. After the semi-finished product passes the inspection , to be filled;
[0046] 4) The semi-finished medicinal liquid is divided into 10ml control bottles according to 5ml per bottle, and the control bottles are placed on the product room plate of the freeze dryer...
Embodiment 2
[0048] 1. Formula (weight: mg): Ganciclovir 250, Dextran 50, Sodium Hydroxide 6.5 and Polysorbate 80 80.
[0049] 2. Preparation method:
[0050] 1) Add the prescribed amount of lactose and dextran into an appropriate amount of water for injection, and stir until completely dissolved to obtain solution 1);
[0051] 2) Dissolve sodium hydroxide in an appropriate amount of water for injection, add ganciclovir and stir, and control the pH value to 11.5 to obtain solution 2);
[0052] 3) Combine solutions 1) and 2), add activated carbon with a prepared volume of 0.5%, boil for 30 minutes, filter and decarbonize, add water for injection to the full amount, and then filter to the storage tank through a sterilizing filter. After the semi-finished product passes the inspection , to be filled;
[0053] 4) The semi-finished medicinal liquid is divided into 10ml control bottles according to 5ml per bottle, and the control bottles are placed on the product room plate of the freeze dryer...
Embodiment 3
[0055] 1. Formula (weight: mg): Ganciclovir 250, Dextran 50, Sodium Hydroxide 6.5 and Polysorbate 80 70.
[0056] 2. Preparation method:
[0057] 1) Add the prescribed amount of lactose and dextran into an appropriate amount of water for injection, and stir until completely dissolved to obtain solution 1);
[0058] 2) Dissolve sodium hydroxide in an appropriate amount of water for injection, add ganciclovir and stir, and control the pH value to 11.5 to obtain solution 2);
[0059] 3) Combine solutions 1) and 2), add activated carbon with a prepared volume of 0.5%, boil for 30 minutes, filter and decarbonize, add water for injection to the full amount, and then filter to the storage tank through a sterilizing filter. After the semi-finished product passes the inspection , to be filled;
[0060] 4) The semi-finished medicinal liquid is divided into 10ml control bottles according to 5ml per bottle, and the control bottles are placed on the product room plate of the freeze dryer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com