Method for preparation of ceftriaxone sodium in vertical logistics system
A technology of ceftriaxone sodium and logistics system, which is applied in the field of pharmacy, can solve the problems of low level of sterility assurance, and achieve the effect of improving the level of sterility assurance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
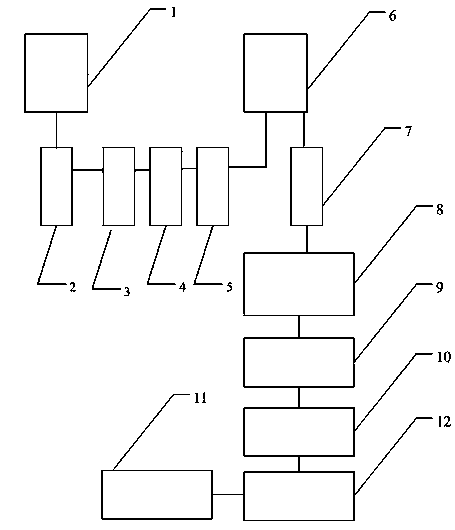
[0023] The present invention will be further described in detail below in conjunction with the accompanying drawings and specific embodiments.
[0024] 1. the method for preparing ceftriaxone sodium in vertical logistic system, specifically comprises the following steps:
[0025] A Preparation of Ceftriaxone Sodium
[0026] Add crude ceftriaxone sodium into acetone solution, fully dissolve at 20-25°C, add seed crystals when the temperature drops to 15°C, and stir until a large amount of crystals are precipitated;
[0027] B crystal drying
[0028] After solid-liquid separation, the obtained solid is transferred from the separation device to the drying device, the separation device is located at the upper end, and the drying device is located at the lower end, and the separation device and the drying device are vertically connected through a connecting channel. In the method for preparing ceftriaxone sodium in a vertical logistics system, a crushing device is vertically conne...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com