Method for large-scale preparation of mixed steam-air sterilized sodium hyaluronate injections
A technology of sodium hyaluronate and air sterilization, applied in the field of biomedicine, can solve the problems of slow dissolution and easy breakage of glycosidic bonds, and achieve the effects of less degradation, stable product quality, and maintaining uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
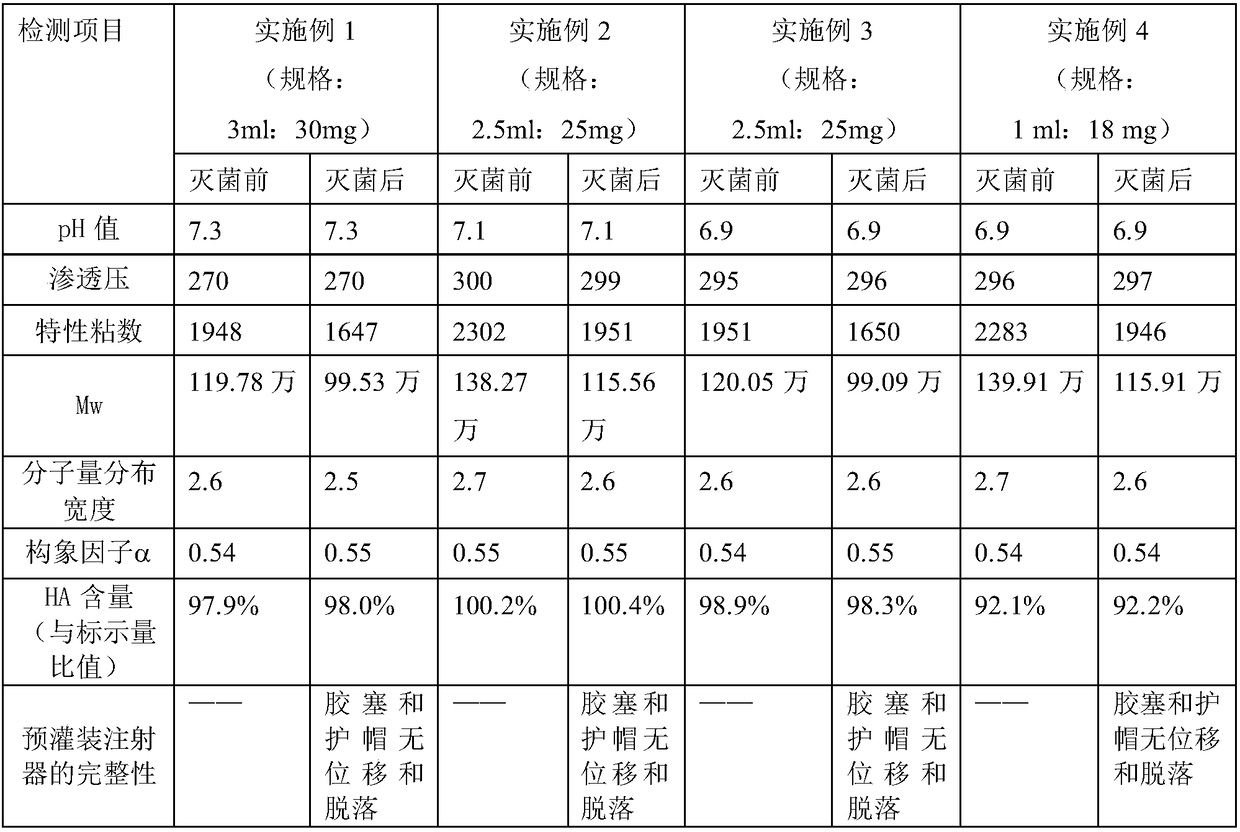
Examples
Embodiment 1
[0021] A method for large-scale preparation of sodium hyaluronate injection mixed with steam-air sterilization, the concrete steps are:
[0022] (1) Physiological buffer preparation:
[0023] Weigh NaCl 1077.18g, NaH 2 PO 4 ·H 2 O 24.31g, Na 2 HPO 4 73.07 g, add 138.1 kg of water for injection, stir and dissolve evenly, then use compressed air to pass 115.1 kg of buffer solution through a 0.22 μm microporous filter element to sterilize and filter to the dissolution tank.
[0024] (2) Preparation and dissolution of sodium hyaluronate injection:
[0025] Get 1151.0 g of sterile sodium hyaluronate bulk drug dry powder (dried and pure) and add it into a dissolving tank by aseptic operation, stir and dissolve for 30 minutes, and let stand for 180 minutes, then stir for 15 minutes every time, let stand for 105 minutes, and after 48 hours, completely dissolved.
[0026] (3) Packing:
[0027] The sodium hyaluronate injection was divided into 3ml pre-filled syringes with a ful...
Embodiment 2
[0032] A method for large-scale preparation of sodium hyaluronate injection mixed with steam-air sterilization, the concrete steps are:
[0033] (1) Physiological buffer preparation:
[0034] Weigh NaCl 294.95.00g, NaH 2 PO 4 ·H 2 O 8.74g, Na 2 HPO 4 16.79 g, add 34.7 kg of water for injection, stir to dissolve, and use compressed air to filter 28.9 kg of buffer solution through a 0.22 μm microporous filter to sterilize and filter to the dissolution tank.
[0035] (2) Preparation and dissolution of sodium hyaluronate injection:
[0036] Get 289.40 g of sterile sodium hyaluronate bulk drug dry powder (dried and pure) and add it into the dissolving tank by aseptic operation, stir and dissolve for 30 minutes, and let it stand for 150 minutes. After that, stir every 10 minutes, let it stand for 90 minutes, and after 30 hours, completely dissolved.
[0037] (3) Packing:
[0038] The sodium hyaluronate injection was dispensed into 3ml pre-filled syringes with a fully automa...
Embodiment 3
[0043] (1) Physiological buffer preparation:
[0044] Weigh 340.00g of NaCl, NaH 2 PO 4 ·H 2 O 4.66g, Na 2 HPO 423.37 g, add 40.0 kg of water for injection, stir to dissolve, and use compressed air to pass 30.0 kg of buffer solution through a 0.22 μm microporous filter to sterilize and filter to the dissolution tank.
[0045] (2) Preparation and dissolution of sodium hyaluronate injection:
[0046] Get 300.14g of sterile sodium hyaluronate bulk drug dry powder (dry and pure), add it into the dissolving tank by aseptic operation, stir and dissolve for 30 minutes, and let stand for 150 minutes, then stir for 10 minutes every time, let stand for 90 minutes, after 30 hours, completely dissolved.
[0047] (3) Packing:
[0048] The sodium hyaluronate injection was dispensed into 3ml pre-filled syringes with a full-automatic vacuum stopper filling machine, each 2.5ml.
[0049] (4) Sterilization:
[0050] Sterilize at 121°C, F0 value is 10.8min, and dry at 105°C for 5min.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com