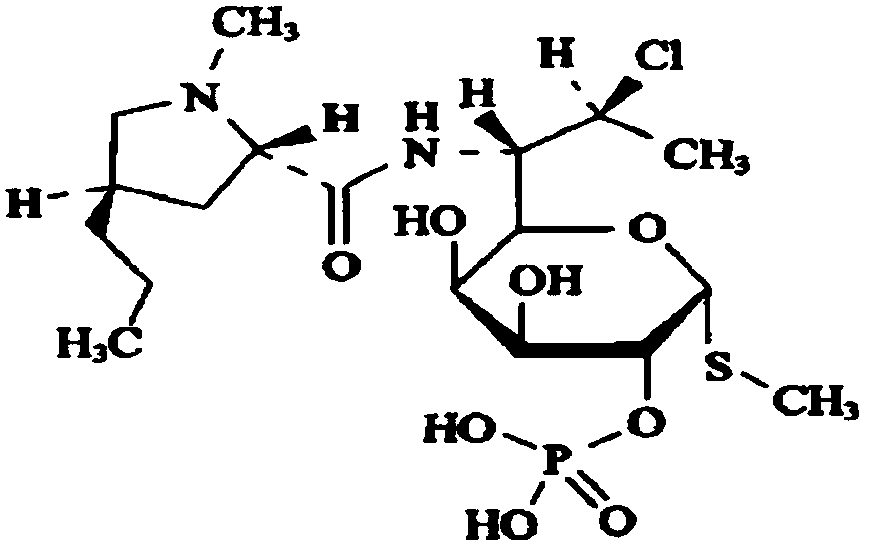
Clindamycin phosphate compound liquid and preparation method thereof
A clindamycin phosphate and liquid preparation technology, which is applied in the field of pharmaceutical inventions, can solve problems such as strong viscosity of clindamycin phosphate, difficulty in ensuring product uniformity, and increased difficulty in dissolving raw materials, so as to improve sterility assurance level, eliminate the oxidation process, and improve the effect of dissolution uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Ingredients: Clindamycin Phosphate 500g, Sodium Hydroxide 35g, Water for Injection 1700g, Activated Carbon 2g.
[0051] Made by any of the following preparation methods:
[0052] Preparation method (1)
[0053] (1) Prepare each ingredient according to the formula ratio;
[0054] (2) Get the sodium hydroxide of formula quantity earlier, add water for injection and be configured into 20% sodium hydroxide solution;
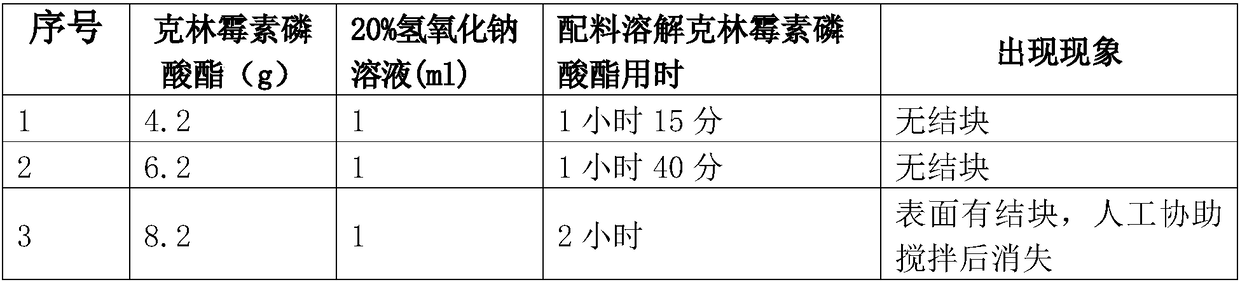
[0055] (3) Turn on the chilled water system, add cooled water for injection into the liquid distribution tank, cool down to 18±2°C, close the chilled water system, and start stirring; 4.2g: Add 1ml of 20% sodium hydroxide solution gradually. After adding the materials, stir until the main ingredient is completely dissolved. Do not lower the temperature during the dissolution process. PH value is 6.1-6.7;
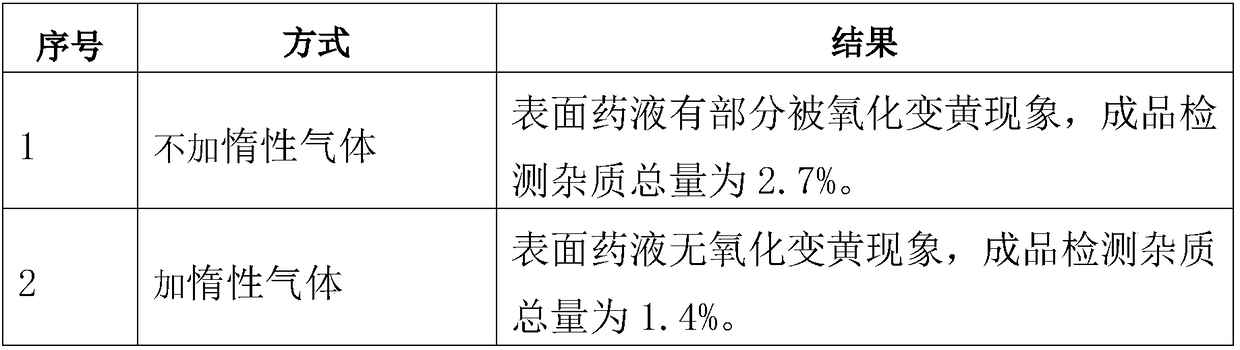
[0056] (4) Add activated carbon, add water for injection to the total amount of the solution, fill the solution tank with nitrogen filtered through a 0....
Embodiment 2
[0074] Ingredients: Clindamycin Phosphate 700g, Sodium Hydroxide 55g, Water for Injection 3000g, Activated Carbon 10g.
[0075] Embodiment 2 is made by any of the following preparation methods
[0076] Preparation method (1)
[0077] (1) Prepare each ingredient according to the formula ratio;
[0078] (2) Get the sodium hydroxide of formula quantity earlier, add water for injection and be configured into 20% sodium hydroxide solution;
[0079] (3) Turn on the chilled water system, add cooled water for injection into the liquid distribution tank, cool down to 18±2°C, close the chilled water system, and start stirring; 4.2g: Add 1ml of 20% sodium hydroxide solution gradually. After adding the materials, stir until the main ingredient is completely dissolved. Do not lower the temperature during the dissolution process. PH value is 6.1-6.7;
[0080] (4) Add activated carbon, add water for injection to the total amount of the solution, fill the solution tank with nitrogen filte...
Embodiment 3
[0098] Ingredients: Clindamycin Phosphate 550g, Sodium Hydroxide 40g, Water for Injection 2000g, Activated Carbon 4g.
[0099] Embodiment 3 is made by any of the following preparation methods
[0100] Preparation method (1)
[0101] (1) Prepare each ingredient according to the formula ratio;
[0102] (2) Get the sodium hydroxide of formula quantity earlier, add water for injection and be configured into 20% sodium hydroxide solution;
[0103] (3) Turn on the chilled water system, add cooled water for injection into the liquid distribution tank, cool down to 18±2°C, close the chilled water system, and start stirring; 4.2g: Add 1ml of 20% sodium hydroxide solution gradually. After adding the materials, stir until the main ingredient is completely dissolved. Do not lower the temperature during the dissolution process. PH value is 6.1-6.7;
[0104] (4) Add activated carbon, add water for injection to the total amount of the solution, fill the solution tank with nitrogen filter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com