Ibuprofen sodium chloride injection preparation with pH of 6.0-6.5, and preparation method thereof
A technology of sodium chloride injection and preparation, which is applied in the direction of anti-inflammatory agents, pharmaceutical formulas, non-central analgesics, etc., and can solve the problems of high dosage of acid-base adjustment, low safety, opalescent opacity, etc., and achieve Increase safety and stability, high safety, reduce the effect of dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of ibuprofen sodium chloride injection (4mg / ml)
[0037] Prescription: Ibuprofen 400g
[0038] L-Arginine 310g
[0039] Sodium chloride 850g
[0040] 0.5mol / L L-arginine appropriate amount
[0041] Water for injection up to 100L
[0042] Makes 1000 bottles
[0043] 1) Obtain qualified ibuprofen raw materials, sodium chloride and L-arginine according to the ingredient list;
[0044] 2) Add water for injection with a batch volume of 4% in the batching tank, add the L-arginine of the batch volume, stir and dissolve, add the ibuprofen of the batch volume, stir for 2 hours, add water for injection to 20% of the batch volume, Add sodium chloride and stir to dissolve, add volume 0.05% (W / V) medicinal activated carbon, stir for 30 minutes, filter with titanium rod, add water for injection to 90% of batch volume, add arginine solution to adjust pH to about 6.3, sti...
Embodiment 2
[0049] Embodiment 2: the preparation of ibuprofen sodium chloride injection (4mg / ml)
[0050] Prescription: Ibuprofen 400g
[0051] L-Arginine 330g
[0052] Sodium chloride 820g
[0053] 0.5mol / L L-arginine appropriate amount
[0054] Water for injection up to 100L
[0055] Makes 1000 bottles
[0056] 1) Obtain qualified ibuprofen raw materials, sodium chloride and L-arginine according to the ingredient list;
[0057] 2) Add water for injection with a batch volume of 4% in the batching tank, add the L-arginine of the batch volume, stir and dissolve, add the ibuprofen of the batch volume, stir for 2 hours, add water for injection to 20% of the batch volume, Add sodium chloride and stir to dissolve, add 0.05% (W / V) medicinal activated carbon by volume, stir for 30 minutes, filter with titanium rod, add water for injection to 90% of batch volume, add arginine solution to adjust pH to about 6.4,...
Embodiment 3
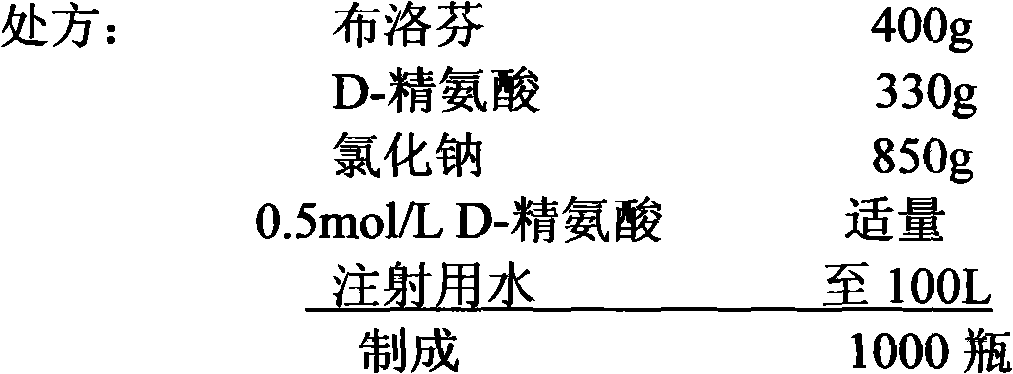
[0062] Embodiment 3: the preparation of ibuprofen sodium chloride injection (4mg / ml)
[0063]
[0064] 1) Obtain qualified ibuprofen raw materials, sodium chloride and D-arginine according to the ingredient list;
[0065] 2) Add water for injection with a batch volume of 4% in the batching tank, add the D-arginine of the batch volume, stir and dissolve, add the ibuprofen of the batch volume, stir for 2 hours, add water for injection to 20% of the batch volume, Add sodium chloride and stir to dissolve, add volume 0.05% (W / V) medicinal activated carbon, stir for 30 minutes, filter with titanium rod, add water for injection to 90% of batch volume, add arginine solution to adjust pH to about 6.3, stir well Finally, add water for injection to the full amount, and fine filter with a cartridge filter;
[0066] 3) After the intermediates pass the test, notify the filling section to start filling;
[0067] 4) Put the filled semi-finished product into the sterilization cabinet for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com