Method of detecting glucose degradation products in buprenorphine hydrochloride injection
A buprenorphine hydrochloride and detection method technology, applied in the field of medicine, can solve the problems affecting product quality evaluation, inability to separate 5-hydroxymethylfurfural from other degradation products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
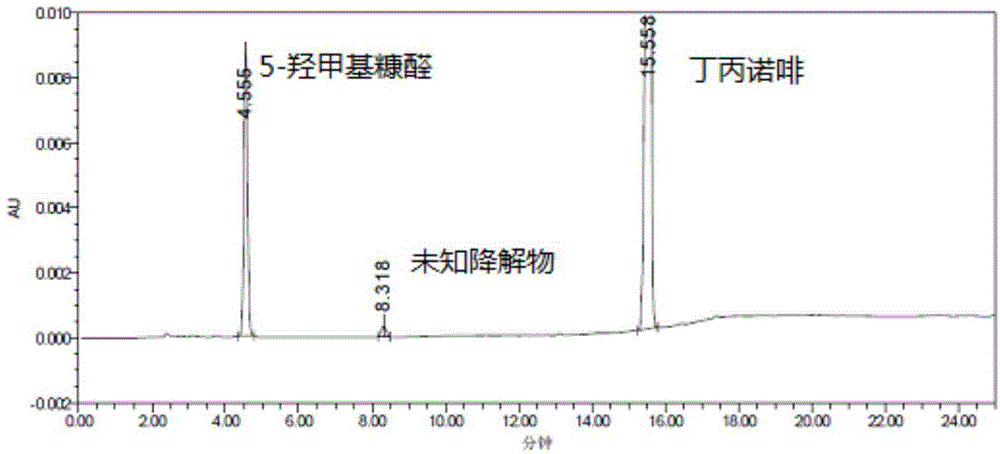
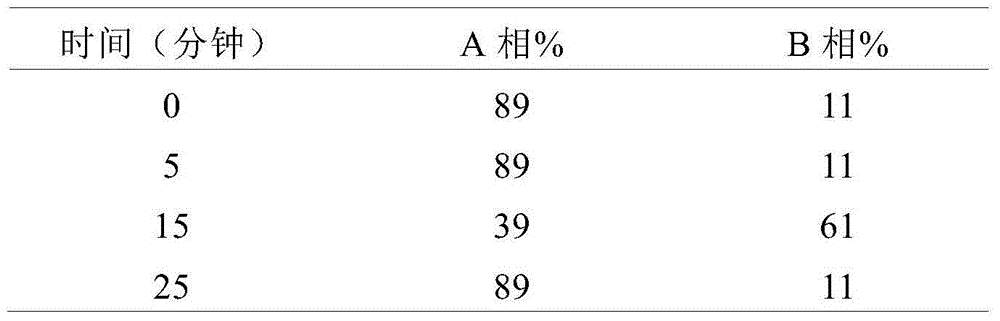
[0019] Chromatographic conditions: ODS chromatographic column is used; the mobile phase A is acetonitrile-potassium dihydrogen phosphate buffer (5.44g potassium dihydrogen phosphate is dissolved in 900ml water, adjusted to pH 4.5 with 5% phosphoric acid, diluted to 1000ml with water), the volume ratio is 10:90, the mobile phase B was acetonitrile, and the gradient elution program was shown in Table 1. The detection wavelength is 284nm, the flow rate is 1.0ml / min, and the column temperature is 30°C.
[0020] Determination method: Take buprenorphine hydrochloride injection and 5% glucose injection to measure according to the above conditions; take another 5-hydroxymethylfurfural reference substance, weigh it accurately, add water to dissolve and dilute to make a solution containing 10 μg per 1ml , as a reference solution.
[0021] The measurement results show that the retention time of 5-hydroxymethylfurfural is about 4.5min, which is much longer than the dead time of the chrom...
Embodiment 2
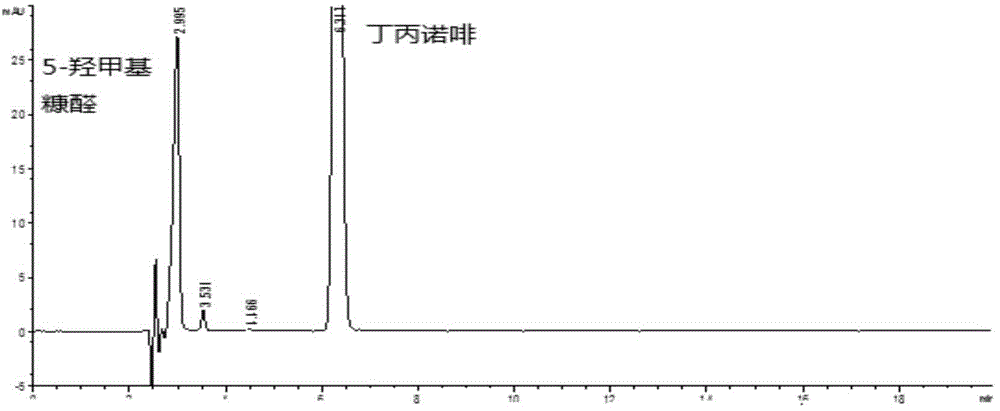
[0023] According to the detection conditions of "5-hydroxymethylfurfural" under the item of buprenorphine hydrochloride injection in the Chinese Pharmacopoeia 2010 edition.
[0024] Chromatographic conditions: ODS column is used; mobile phase is methanol-acetonitrile-2% ammonium acetate solution-glacial acetic acid (60:10:40:5); flow rate is 1.0ml / min; detection wavelength is 288nm; column temperature is 30°C .
[0025] Determination method: Take buprenorphine hydrochloride injection and 5% glucose injection to measure according to the above conditions; take another 5-hydroxymethylfurfural reference substance, weigh it accurately, add water to dissolve and dilute to make a solution containing 10 μg per 1ml , as a reference solution.
[0026] The measurement results showed that the retention time of 5-hydroxymethylfurfural was about 3 minutes, which was close to the solvent peak, and the separation was not complete, and other degradation products of glucose were not separated,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com