Process for production of buprenorphine pharmaceutical preparation to be applied to mouth mucosa
A technology for oral mucosa and buprenorphine hydrochloride, which is applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, pill delivery, etc., and can solve the problem of decomposition of preparations, poor stability of buprenorphine, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
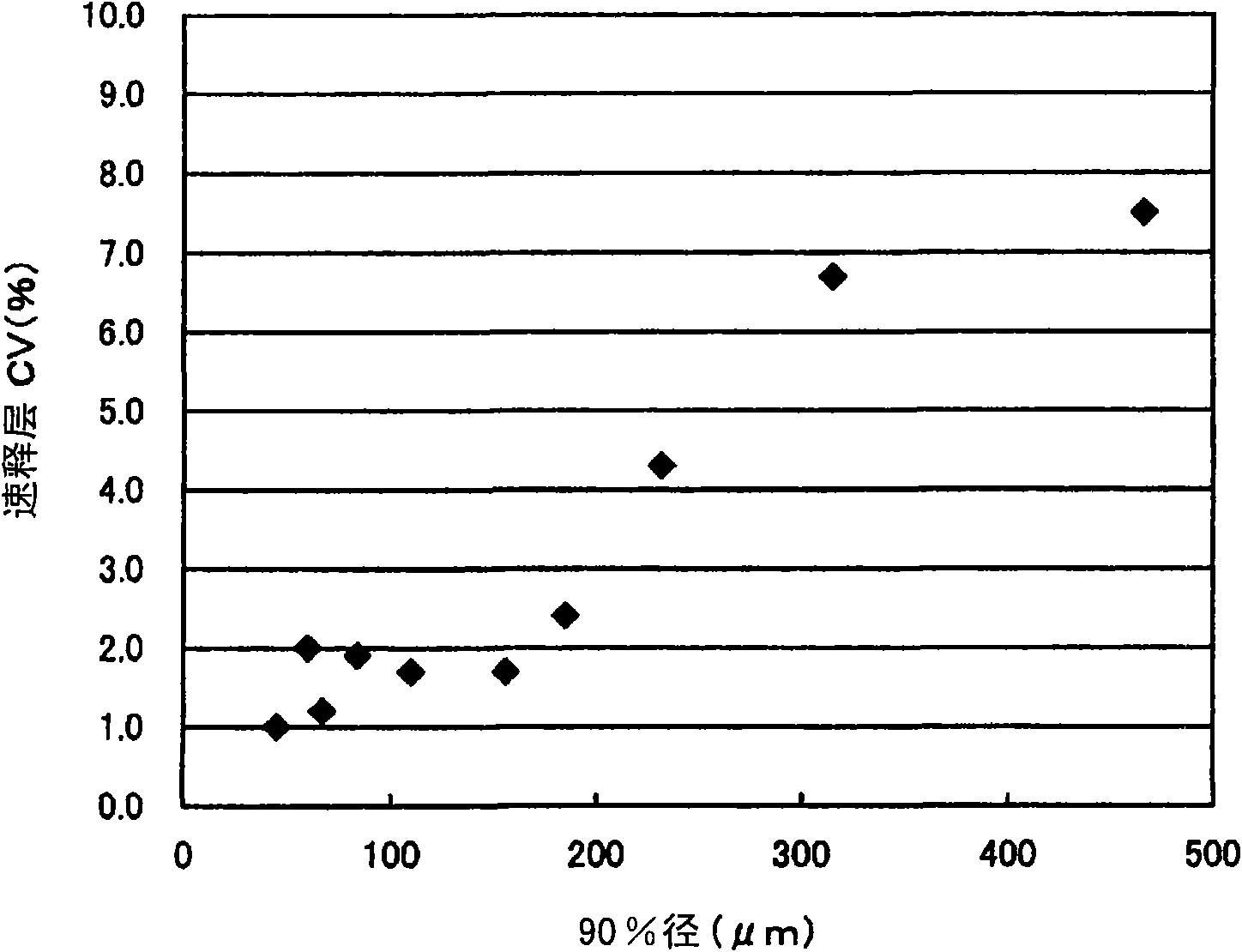
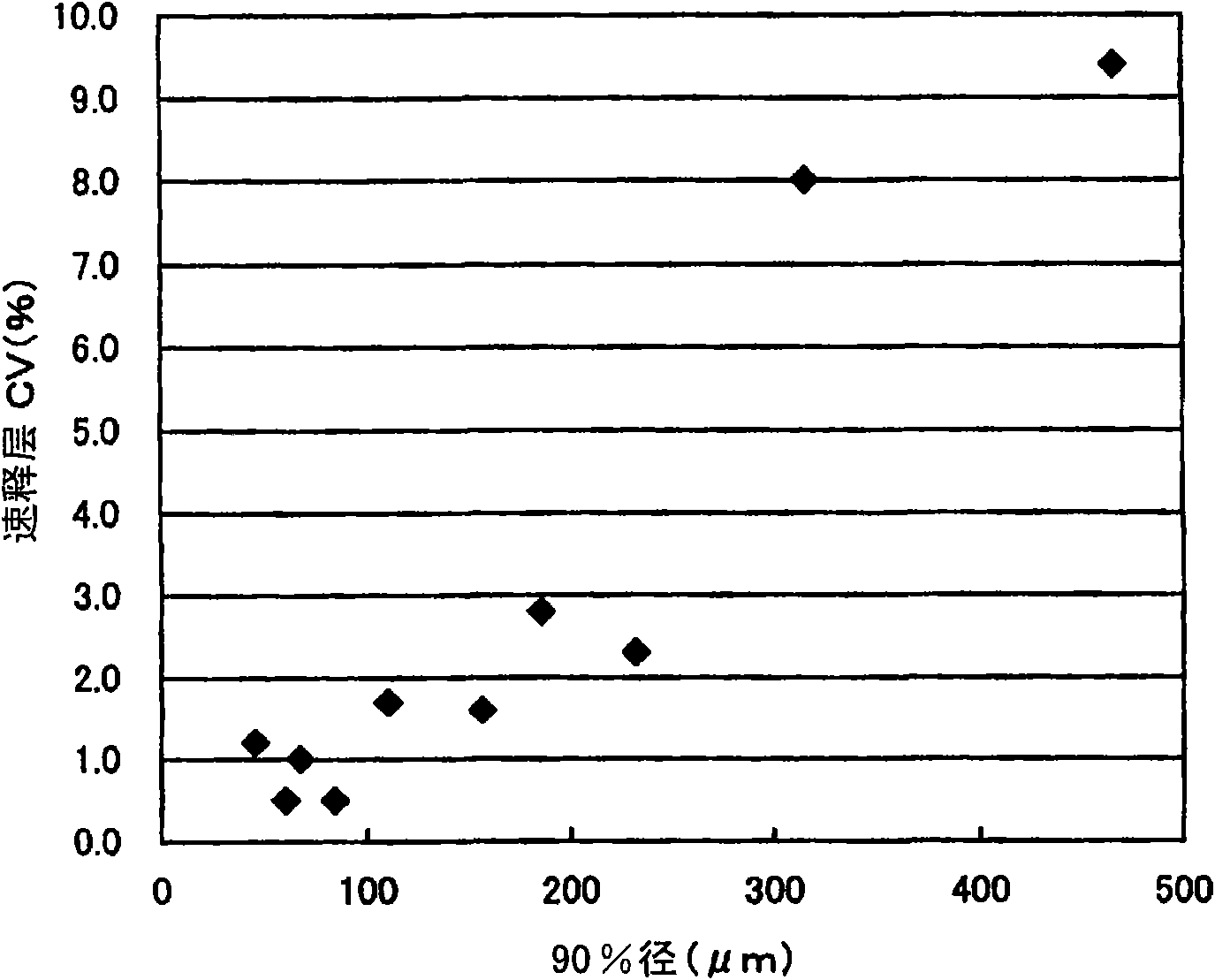
[0091] Buprenorphine hydrochloride crystals were pulverized four times with a hammer mill to obtain fine particles with a 50% cumulative diameter of 25 μm and a 90% cumulative diameter of 61 μm. Granules for a sustained-release layer were prepared in the same manner as in Comparative Example 2 (2) except that the buprenorphine hydrochloride crystals were used.
Embodiment 2
[0093] The buprenorphine hydrochloride crystals used in Comparative Example 2 above were sieved so that the 50% cumulative diameter was 23 μm and the 90% cumulative diameter was 48 μm. Using the buprenorphine hydrochloride crystals, except that the content of buprenorphine hydrochloride was halved, a bilayer tablet for buprenorphine hydrochloride for oral mucosal adhesion was prepared in the same manner as in Comparative Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com