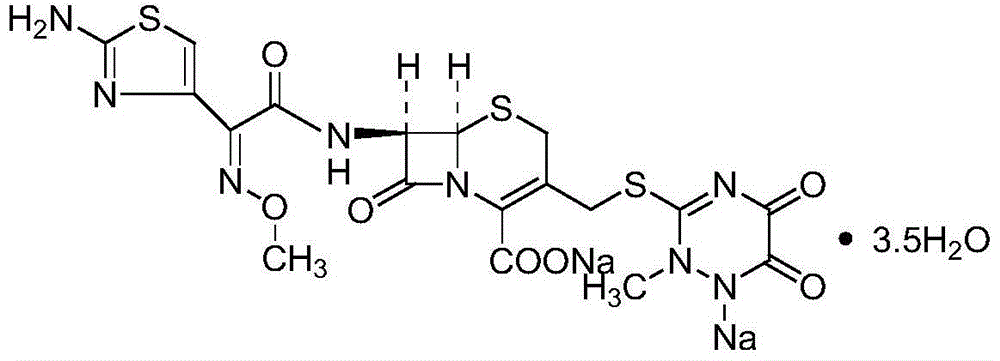
Children pharmaceutical composition containing ceftriaxone sodium and low-sodium carrier
A technology of ceftriaxone sodium and composition, which is applied in the field of children's ceftriaxone sodium and low-sodium carrier pharmaceutical composition, can solve the problems of poor stability of ceftriaxone sodium, occurrence of degradation products, easy discoloration of appearance, etc. Clinical application quality, less impurities, and the effect of reducing impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
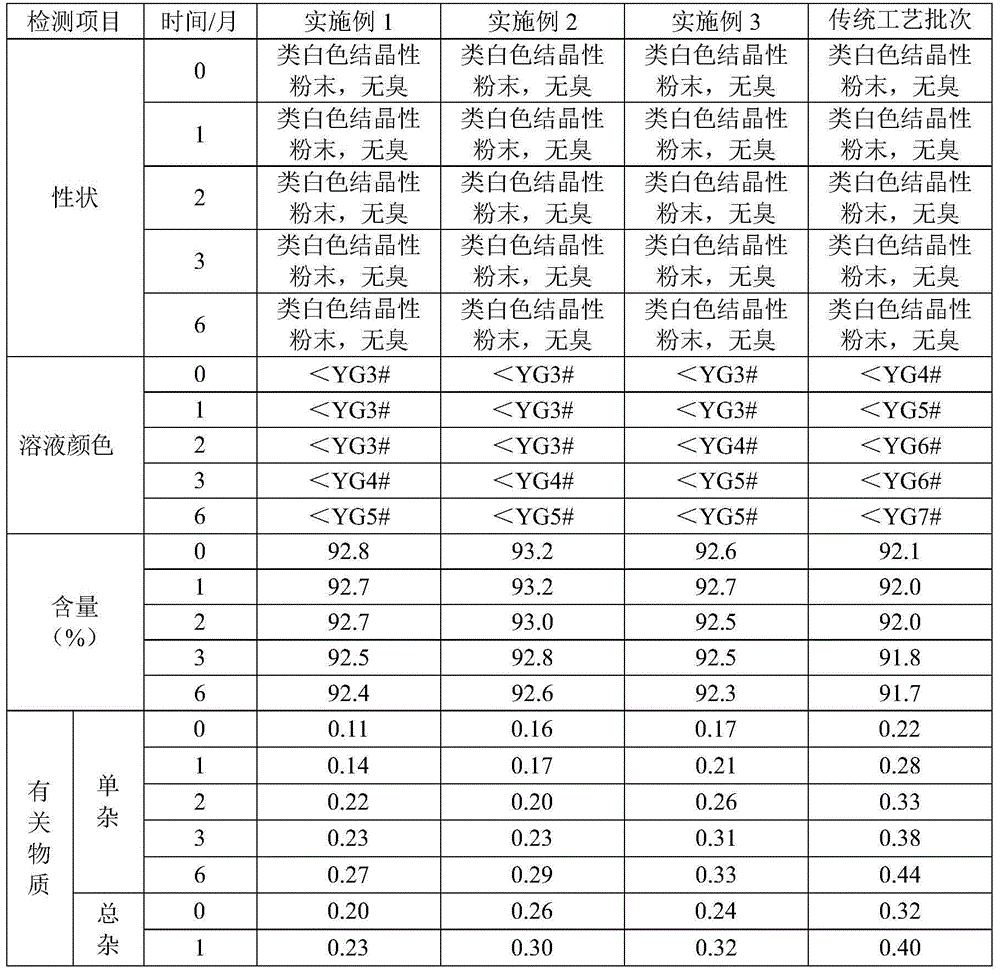
Examples
Embodiment 1
[0037] (1) Weigh 100g of ceftriaxone crude product, add 1000ml of water, heat up to 30°C until completely dissolved, add 10ml of ethyl acetate under stirring, transfer to a 1000ml pressure-resistant container, ensure that it is full and remove air bubbles, seal the container, oscillate, and control Take out after freezing at -18°C for 8 hours;
[0038] (2) Liquid-solid separation, after the ice melts, add 10g of activated carbon, stir and decolorize, filter;
[0039] (3) Transfer the filtrate to a crystallization tank, control the temperature at 10-15°C, add 5000ml of acetone dropwise for about 1 hour under the protection of nitrogen, stir at a slow speed for 30min, continue to grow crystals for 1h, filter with suction, wash with acetone, and dry in vacuo at 40°C; Preparations of different specifications (0.25g / bottle, 0.5g / bottle, 1.0g / bottle, 2.0g / bottle) are sub-packaged, and the temperature and humidity of the controlled environment are 20-24°C, and the humidity is less th...
Embodiment 2
[0041] (1) Weigh 100g of ceftriaxone crude product, add 1000ml of water, heat up to 30°C until completely dissolved, add 10ml of chloroform under stirring, transfer to a 1000ml pressure-resistant container, ensure that it is full and remove air bubbles, seal the container, oscillate, and control the temperature- Freeze at 18°C for 3 hours and take out;
[0042] (2) liquid-solid separation, the liquid phase removes the organic phase, and after the ice melts, the water phase is combined, 10 g of activated carbon is added, stirred for decolorization, and filtered;
[0043] (3) Transfer the filtrate to a crystallization tank, control the temperature at 10-15°C, add 5000ml of acetone dropwise for about 1 hour under the protection of nitrogen, stir at a slow speed for 30min, continue to grow crystals for 1h, filter with suction, wash with acetone, and dry in vacuo at 40°C; Preparations of different specifications (0.25g / bottle, 0.5g / bottle, 1.0g / bottle, 2.0g / bottle) are sub-packag...
Embodiment 3
[0045] (1) Weigh 80g of ceftriaxone crude product, add 1000ml of water, heat up to 30°C until completely dissolved, add 5ml of ethyl acetate under stirring, transfer to a 1000ml pressure-resistant container, ensure that it is full and remove air bubbles, seal the container, oscillate, and control Take out after freezing at -18°C for 8 hours;
[0046] (2) Liquid-solid separation, after the ice melts, add 10g of activated carbon, stir and decolorize, filter;
[0047] (3) Transfer the filtrate to a crystallization tank, control the temperature at 10-15°C, add 5000ml of acetone dropwise for about 1 hour under the protection of nitrogen, stir at a slow speed for 30min, continue to grow crystals for 1h, filter with suction, wash with acetone, and dry in vacuo at 40°C; Preparations of different specifications (0.25g / bottle, 0.5g / bottle, 1.0g / bottle, 2.0g / bottle) are sub-packaged, and the temperature and humidity of the controlled environment are 20-24°C, and the humidity is less than...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com