Production technology of glucose sodium chloride injection
A glucose sodium chloride and production process technology, which is applied in the field of glucose sodium chloride injection production technology, can solve the problem of affecting the production efficiency and quality safety of glucose sodium chloride injection, the residue of activated carbon particles and other particles, and the residue of activated carbon Particles cannot be completely removed, so as to prevent dust particles and sedimentation bacteria from entering the liquid medicine, reduce the content of particles and endotoxins, and prevent sugars from being oxidized
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A production process for glucose and sodium chloride injection, comprising the following steps:
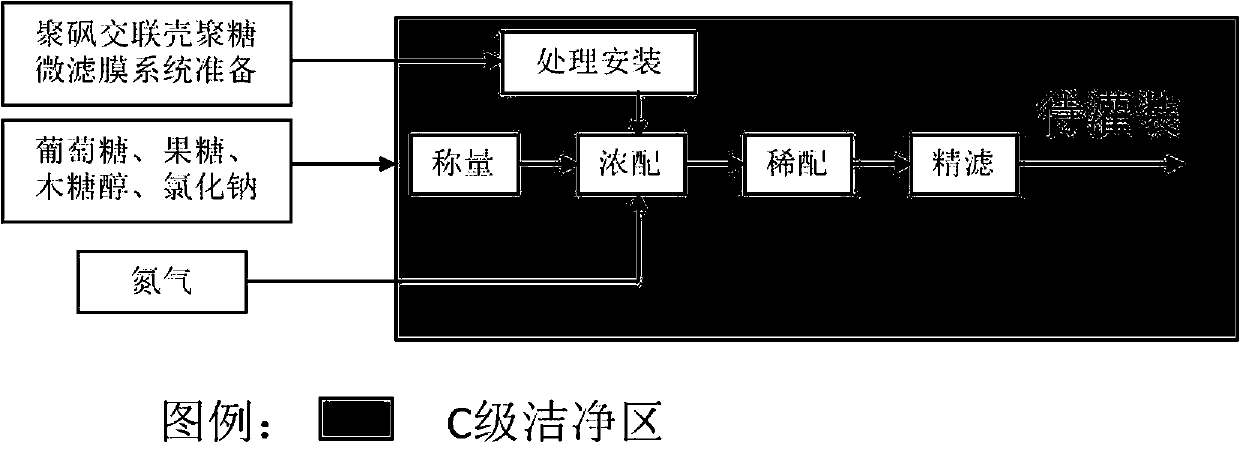
[0043] Step 1. Weighing: Calculate the required amount of glucose, fructose, xylitol and sodium chloride according to the total amount of preparation, and then accurately weigh, one person weighing and one person reviewing;
[0044] Step 2. Concentrated preparation: add 25% water for injection to the concentrated preparation tank, control the temperature at 80±5 °C, pass nitrogen, put glucose, fructose, xylitol and sodium chloride into it, pass nitrogen insulation conditions Under the stirring cycle for 30 minutes, it was filtered through the polysulfone cross-linked chitosan microfiltration membrane system, and the foreign matter in the liquid was checked to be qualified;
[0045] Step 3. Dilute preparation: rinse the concentrated preparation tank and the drug delivery pipeline three times with about 1000L water for injection, the flushing water is filtered and sent to the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com