Child type piperacillin tazobactam sodium and low-sodium carrier pharmaceutical composition
A technology of tazobactam sodium and piperacillin sodium, which is applied in the direction of drug delivery, active ingredients of heterocyclic compounds, antibacterial drugs, etc., can solve the problems of degradation products, easy discoloration of appearance, and content reduction, and achieve simplified steps , Improve the quality of clinical application, the effect of small toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
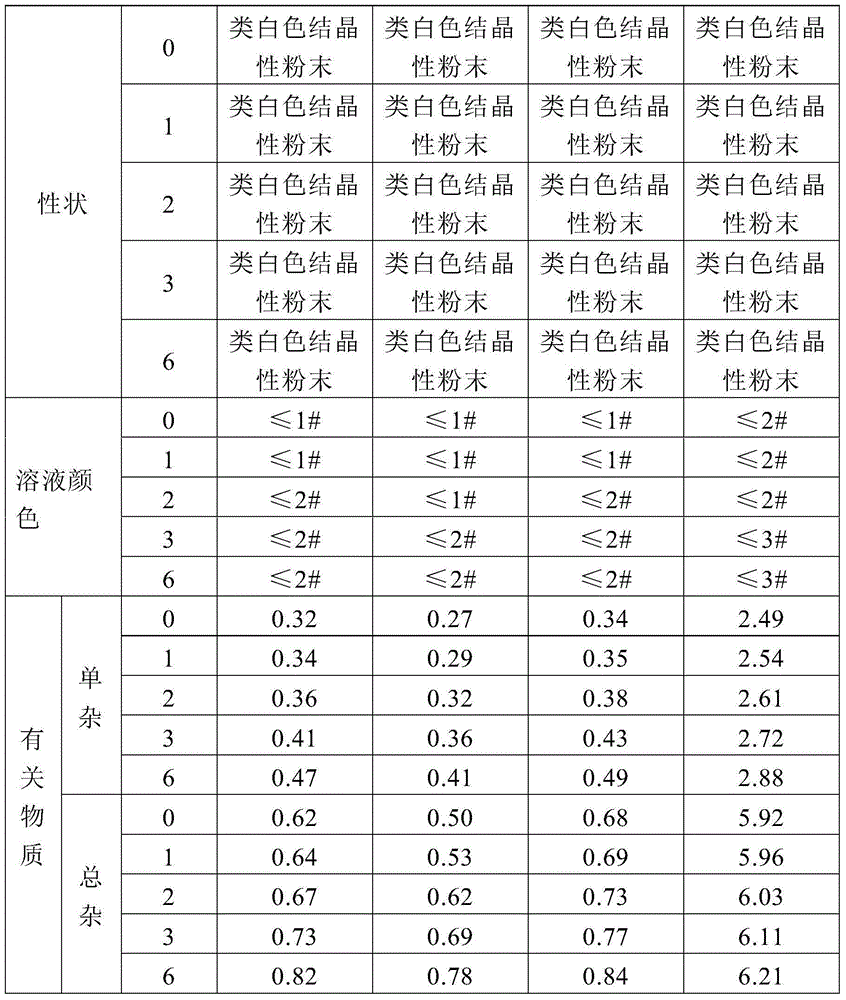
Examples
Embodiment 1
[0044] Add 1523ml of acetone in the reactor, add 120 grams of piperacillin, stir and dissolve, add gac, stir for 20 minutes, filter, the filtrate is added dropwise sodium acetate ethanol solution, sodium acetate ethanol solution consists of 20.2 grams of sodium acetate, 70.8ml of purified water and 384ml of absolute ethanol was mixed and dissolved to form, after the dropwise addition was completed, the reaction was incubated for 2 hours, filtered, the filter cake was washed with acetone, and vacuum-dried to obtain 111.4 grams of piperacillin sodium crude product.
[0045] Weigh 100 g of the crude piperacillin sodium, add 1000 ml of purified water, heat up to 30°C until completely dissolved, add 10 g of activated carbon, stir to decolorize, and filter to obtain the filtrate.
[0046] Add 5ml of ethyl acetate to the above filtrate under stirring, transfer to a 1000ml pressure-resistant container, make sure it is full and the air bubbles are removed, seal the container, oscillate,...
Embodiment 2
[0050] Add 1523ml of acetone in the reactor, add 120 grams of piperacillin, stir and dissolve, add gac, stir for 20 minutes, filter, the filtrate is added dropwise sodium acetate ethanol solution, sodium acetate ethanol solution consists of 20.2 grams of sodium acetate, 70.8ml of purified water and 384ml of absolute ethanol was mixed and dissolved to form, after the dropwise addition was completed, the reaction was incubated for 2 hours, filtered, the filter cake was washed with acetone, and vacuum-dried to obtain 111.4 grams of piperacillin sodium crude product. .
[0051] Weigh 100 g of the crude piperacillin sodium, add 500 ml of purified water, heat up to 30°C until completely dissolved, add 7 g of activated carbon, stir to decolorize, and filter to obtain the filtrate.
[0052]Add 5ml of chloroform to the above filtrate under stirring, transfer it to a 500ml pressure-resistant container, make sure it is full and the air bubbles are removed, seal the container, oscillate, ...
Embodiment 3
[0056] Add 1523ml of acetone in the reactor, add 120 grams of piperacillin, stir and dissolve, add gac, stir for 20 minutes, filter, the filtrate is added dropwise sodium acetate ethanol solution, sodium acetate ethanol solution consists of 20.2 grams of sodium acetate, 70.8ml of purified water and 384ml of absolute ethanol was mixed and dissolved to form, after the dropwise addition was completed, the reaction was incubated for 2 hours, filtered, the filter cake was washed with acetone, and vacuum-dried to obtain 111.4 grams of piperacillin sodium crude product.
[0057] Weigh 100 g of crude piperacillin sodium, add 350 ml of purified water, heat up to 30°C until completely dissolved, add 3 g of activated carbon, stir to decolorize, and filter to obtain the filtrate.
[0058] Add 2.3ml of ethyl acetate and 2.3ml of chloroform mixed solvent to the above filtrate under stirring, transfer to a 350ml pressure-resistant container, make sure it is full and air bubbles are removed, s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com