Pharmaceutical cefotiam composition for treating infectious diseases
An infectious disease, cefotiam hydrochloride technology, applied in the field of medicine, can solve problems such as poor mixing uniformity, unstable β-lactam ring, and instability of cefotiam hydrochloride for injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
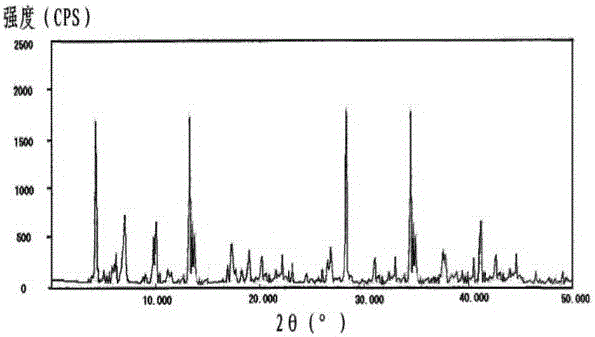
[0027] Example 1: Preparation of Cefotiam Hydrochloride Crystals
[0028] (1) Grind the crude product of cefotiam hydrochloride, pass it through a 60-mesh sieve, then add it into deionized water whose volume is 6 times the weight of cefotiam hydrochloride, and stir at 130 rpm for 10 minutes;
[0029] (2) Add ethanol whose volume is 4 times the weight of cefotiam hydrochloride under stirring at 90 rpm, and raise the temperature to 30°C at the same time;
[0030] (3) After adding the solution, let it stand for 3 hours, add dropwise a mixed solution of ether and chloroform whose volume is 8 times the weight of cefotiam hydrochloride at 0°C under stirring at 150 rpm, the volume of ether and chloroform The ratio is 3:1, and the dropwise addition is completed within 2 hours;
[0031] (4) After the dropwise addition was completed, the temperature was lowered to -5°C, and the stirring rate was continued at 110 rpm for 2 h, and the crystals were precipitated after standing for 1 h, ...
Embodiment 2
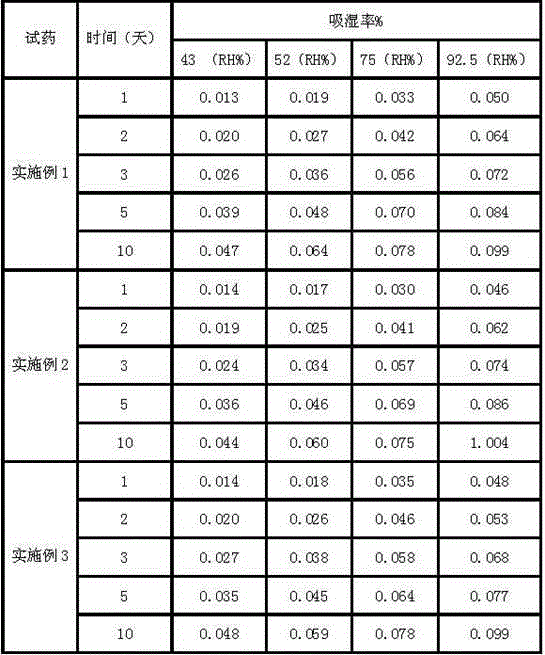
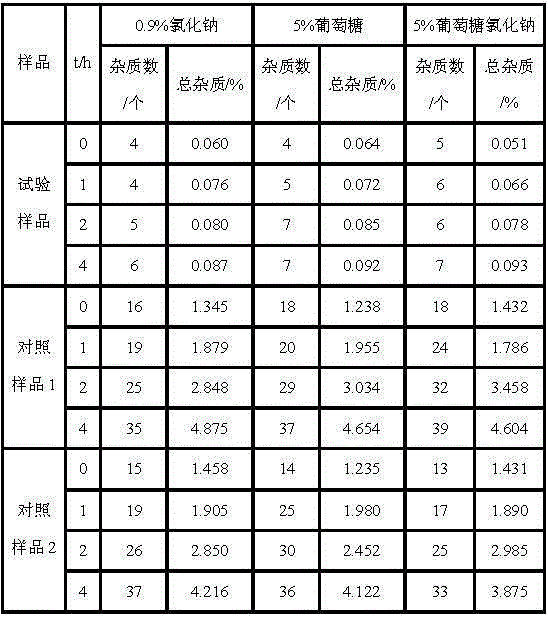
[0033] Example 2: The preparation method of cefotiam hydrochloride composition
[0034] The composition comprises: 1 part by weight of cefotiam hydrochloride crystal prepared by the present invention, and 0.1 part by weight of anhydrous sodium carbonate.
[0035] The preparation method is:
[0036] (1) Weigh cefotiam hydrochloride crystals and anhydrous sodium carbonate in proportion and mix them thoroughly;
[0037] (2) Dispense into sterilized vials and stopper them.
Embodiment 3
[0038] Example 3: Preparation of cefotiam hydrochloride composition
[0039] The composition comprises: 1 part by weight of cefotiam hydrochloride crystal prepared by the present invention, and 0.15 part by weight of anhydrous sodium carbonate.
[0040] The preparation method is:
[0041] (1) Weigh cefotiam hydrochloride crystals and anhydrous sodium carbonate in proportion and mix them thoroughly;
[0042] (2) Dispense into sterilized vials and stopper them.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com